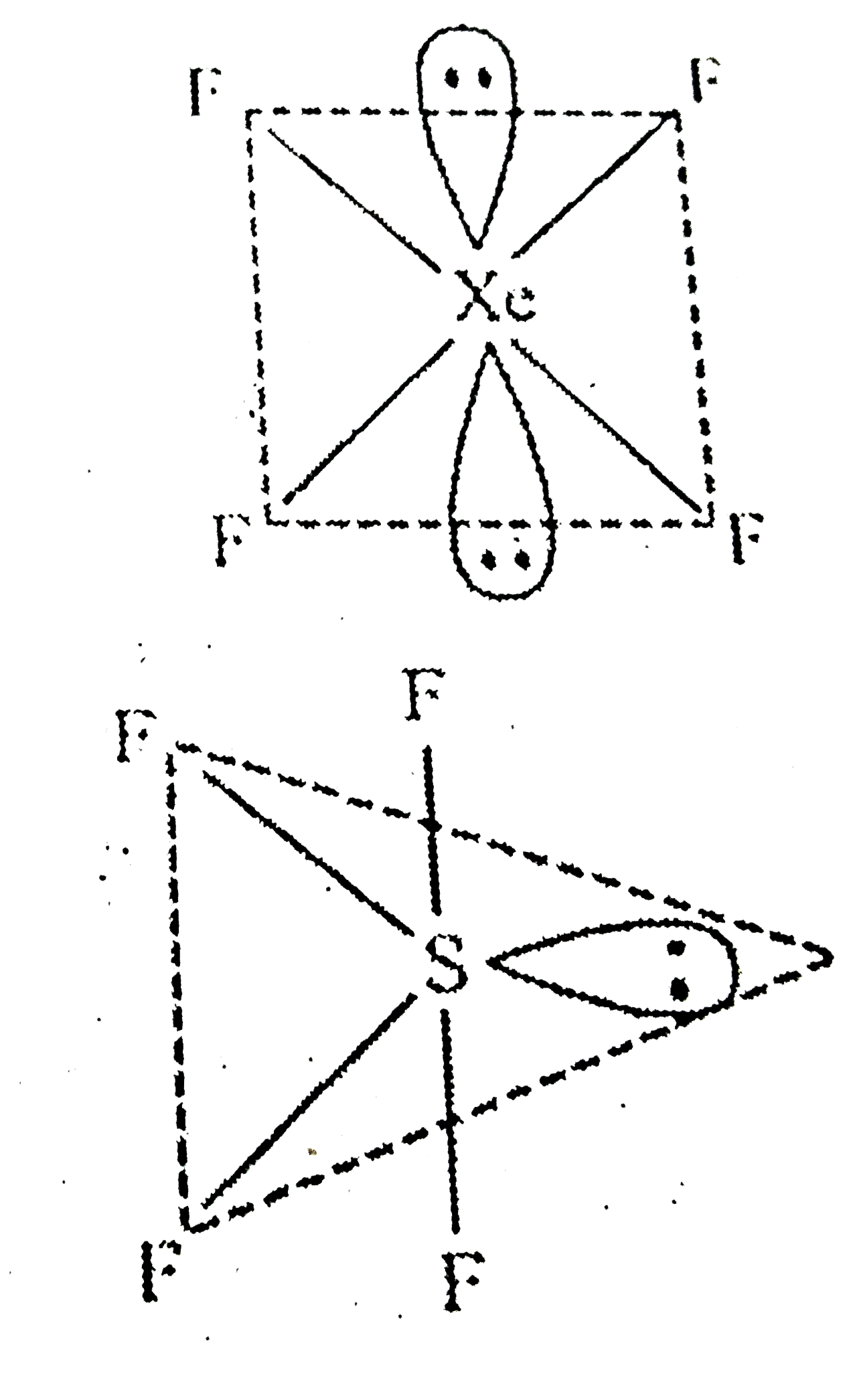A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
AIIMS PREVIOUS YEAR PAPERS-AIIMS 2006-CHEMISTRY
- The ligands in anti- cancer drug cisplatin are
Text Solution
|
- Given below, catalyst and corresponding process/reaction are matched. ...
Text Solution
|
- Among the following , the species having square planar geometry for ce...
Text Solution
|
- Tincture iodine is :
Text Solution
|
- In [Ag(CN)2]^(-), the number of pi bonds is
Text Solution
|
- The compound which has molecular nature in gas phase but ionic in soli...
Text Solution
|
- Which two of the following salts are used for preparing iodized salt? ...
Text Solution
|
- The compound used in enrichment of uranium for nuclear power plant is
Text Solution
|
- The de Broglie wavelength associated with a ball of mass 1kg having ki...
Text Solution
|
- Dominance of strong repulsive forces among the molecules of the gas (Z...
Text Solution
|
- 40 ml of 0.1 M ammonia is mixed with 20 ml of 0.1 M HCI. What is the p...
Text Solution
|
- For a spontaneous process the correct statement is -
Text Solution
|
- The Ca^(2+) and F^(-) ions arc located in CaF(2) crystal respectively ...
Text Solution
|
- The charge required for the reduction of 1 mol of MnO(4)^(-) to MnO(2)...
Text Solution
|
- For the reaction 2N(2)O(5 )rarrNO(2)+O(2) rate of reaction is :
Text Solution
|
- For a phase change: H(2)O(l)hArrH(2)O(s) 0^(@)C, 1 bar
Text Solution
|
- A 5% solution (by mass) of cane sugar in water has freezing point of 2...
Text Solution
|
- The energy gaps (E(g)) between valence band and conduction band for di...
Text Solution
|
- The enthalpy change (Delta H) for the reaction N(2) (g) + 3 H(2) (g)...
Text Solution
|
- The products formed when an aqueous solution of NaBr is electrolysed i...
Text Solution
|