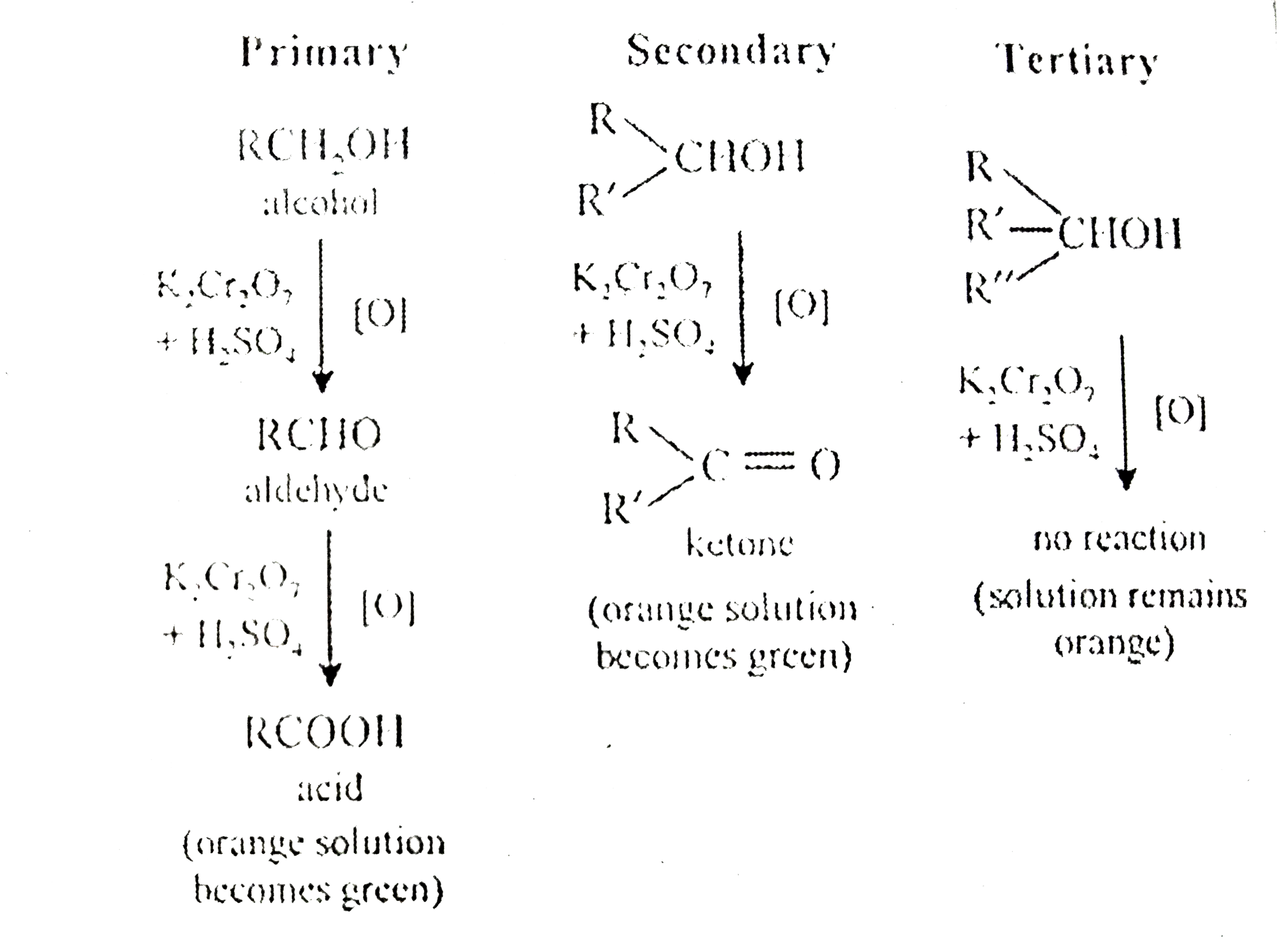
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
AIIMS PREVIOUS YEAR PAPERS-AIIMS 2006-CHEMISTRY
- Assertion: In the iodometric titration, starch is used as an indicator...
Text Solution
|
- Assertion : Nitrogen is less reactive than molecular oxygen. Reason:...
Text Solution
|
- These questions consist of two statements each, printed as Assertion a...
Text Solution
|
- Assertion: E^(@) for Mn^(3+)//Mn^(2+) is more positive than Cr^(3+)//C...
Text Solution
|
- Assertion: K2Cr2O7 is used as primary standard in volumetric analysis....
Text Solution
|
- Assertion: Silicones are hydrophobic in nature. Reason: Si-O-Si link...
Text Solution
|
- According to the tranistion state theory, for the formation of on acti...
Text Solution
|
- Assertion :- Water in liquid state is more stable than ice at room tem...
Text Solution
|
- Assertion : Sb(2)S(3) is not soluble in yellow ammonium sulphide. Re...
Text Solution
|
- Assertion (A) : Graphite is an example of tetragonal crystal system. ...
Text Solution
|
- Assertion : For the Daniel cell, Zn//Zn^(2+)||Cu^(2+)|Cu" with "E(cell...
Text Solution
|
- Assertion : Fe^(3+) ions can be used for the coagulation of As(2)S(3) ...
Text Solution
|
- Each question contains STATEMENT-I(Assertion) and STATEMENT-2(Reason)....
Text Solution
|
- Assertion : Change in colour of the acidic solution of breath is used ...
Text Solution
|
- Assertion : Anilinium choride is more acidic than ammonium chloride. ...
Text Solution
|
- Assertion: Diastereoisomers have different physical properties. Reas...
Text Solution
|
- Assertion : The presence of nitro group facilitates nucleophilic subs...
Text Solution
|
- Assertion : 1,3- Butadiene is the monomer for natural rubber. Reason...
Text Solution
|
- A) Addition of HBr on 2-butene gives two isomeric products. R) Addit...
Text Solution
|
- Statement - The water pouch of instant cold pack for treating athlet...
Text Solution
|