A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
AIIMS PREVIOUS YEAR PAPERS-AIIMS 2007-CHEMISTRY
- Critical temperatures for A, B, C and D gases are 25^(@)C, 10^(@)C, -8...
Text Solution
|
- Which of the following metal ions will form complexes with the same ma...
Text Solution
|
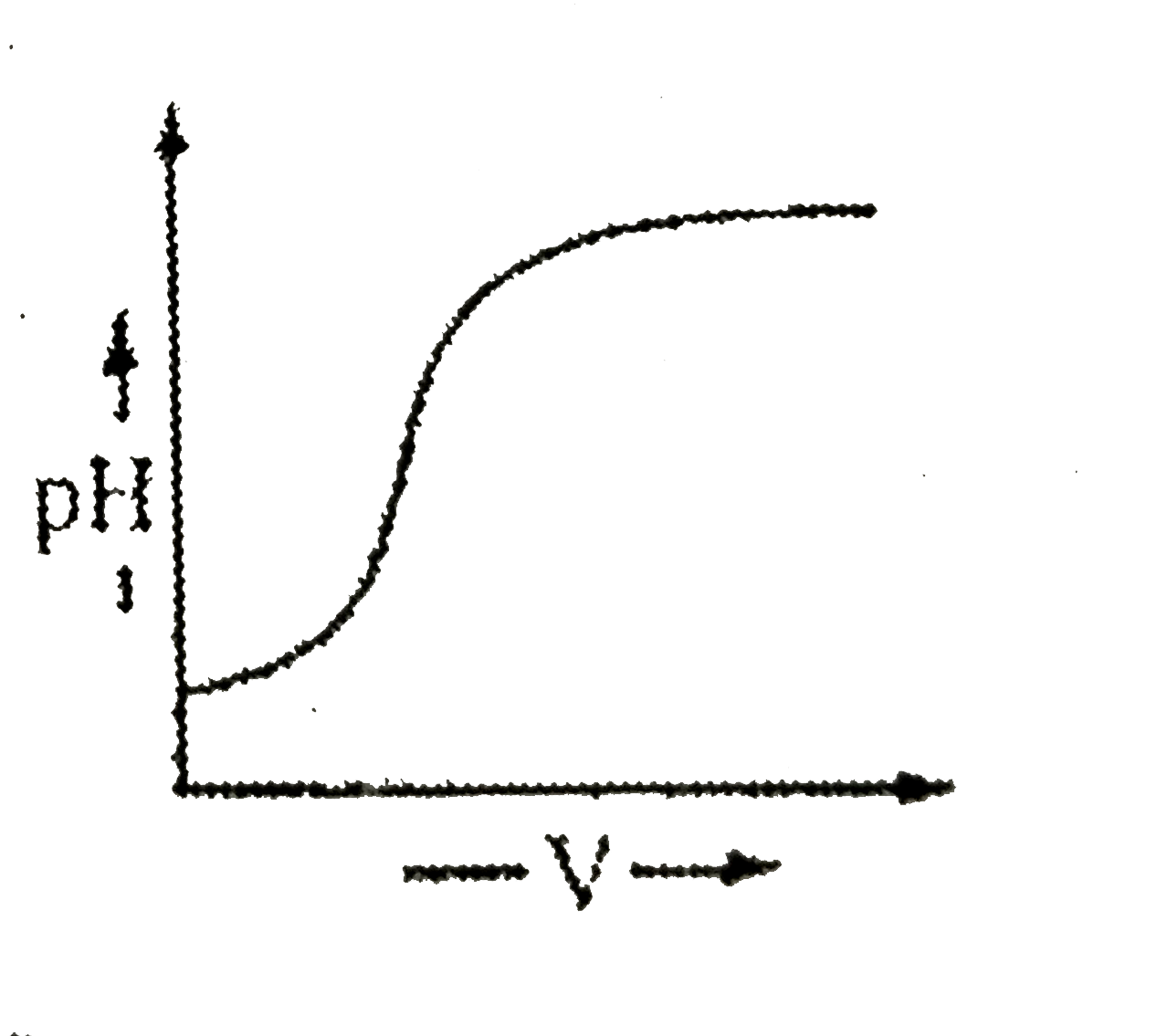
- During titration of acetic acid with aq. NaOH solution, the neutralisa...
Text Solution
|
- Which of the following radioistopes is used as anticancerous ?
Text Solution
|
- XeF(6) on complete hydrolysis gives
Text Solution
|
- Calculate change in internal energy if Delta H= - 92.2 kJ, P = 40 atm ...
Text Solution
|
- Delta H("fusion") of a substance is 'x' and Delta H("vap") is 'y', the...
Text Solution
|
- Decay constant of a radioactive substance is 69.3 sec^(-1), find t(1//...
Text Solution
|
- The repeating structural unit of silicone is
Text Solution
|
- Propene on hydroboration and oxidation produces
Text Solution
|
- on mercuration and demercuration produces
Text Solution
|
- The product obtained is//are
Text Solution
|
- The element which is the most abundant in the earth crust is
Text Solution
|
- Wavelength of red light is absorbed by the complex
Text Solution
|
- In the change [Cu(H(2)O)(6)]^(2+)overset(HCl)rarr [CuCl(H(2)O)(5)]^(+)...
Text Solution
|
- Benzoic acid is treated with lithium aluminium hydribve. The compound ...
Text Solution
|
- Chain transfer reagent is:
Text Solution
|
- Among the following components of cement which is present in highest a...
Text Solution
|
- A catalyst
Text Solution
|
- Which of the following does not contain a coordinate bond?
Text Solution
|