A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
AIIMS PREVIOUS YEAR PAPERS-AIIMS 2007-CHEMISTRY
- Which statement is true for ferrocene ?
Text Solution
|
- Assertion : Copper sulphate solution is not stored in zinc vessel. ...
Text Solution
|
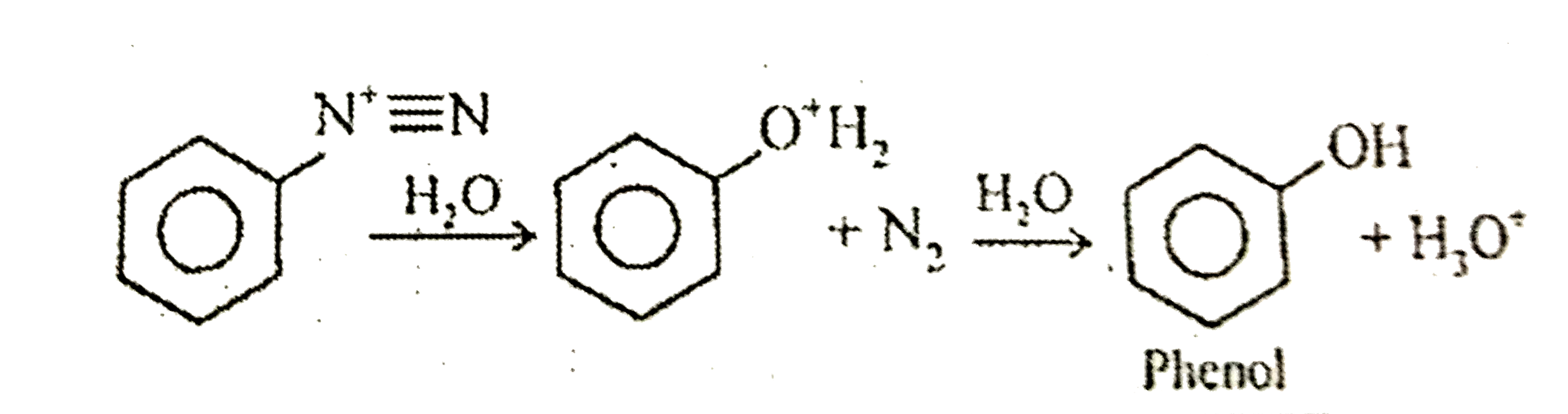
- Assertion : Benzene diazonium salt on boiling with water forms phenol....
Text Solution
|
- Assertion : trans-butene on reaction with bromine forms recemic mixtur...
Text Solution
|
- Assertion : Ozone is an allotrope of oxygen. Reason : Oxygen is blui...
Text Solution
|
- Assertion (A) : SnI4 is an orange solid . Reason (R) : The colour ...
Text Solution
|
- Assertion: Acetamide has more polargt C = 0 group than ethyl acetoacet...
Text Solution
|
- Assertion : Magnetic moment of Dy is the highest among langthanoids. ...
Text Solution
|
- Assertion : C - O bond in metal carbonyl is long. Reason : There is ...
Text Solution
|
- Assertion : Chloral reacts with phenyl chloride to form DDT. Reason ...
Text Solution
|
- Assertion : Mixture of CH(3)COOH and CH(3)COONH(4) is an example of ac...
Text Solution
|
- Assertion : Alkyl iodide can be prepared by treating alkyl chloride/br...
Text Solution
|
- Assertion: F is more electronegative than Cl. Reason: F has high ele...
Text Solution
|
- Assertion : Asetylene on reacting with sodamide gives sodium acetylide...
Text Solution
|
- Assertion(A) : When sodium chloride dissolves in water, then Na^(+) an...
Text Solution
|
- Assertion : Alpha(alpha)- amino acids exist as internal salt in soluti...
Text Solution
|
- Assertion : The kinetics of the reaction - mA + nB + pC rarr m'X + ...
Text Solution
|
- Assertion : Nitrogen is less reactive than molecular oxygen. Reason:...
Text Solution
|
- Assertion : The lactic acid shows the geometrical isomerism. Reaso...
Text Solution
|
- Assertion (A) : The equilibrium constant is fixed and characteristic f...
Text Solution
|