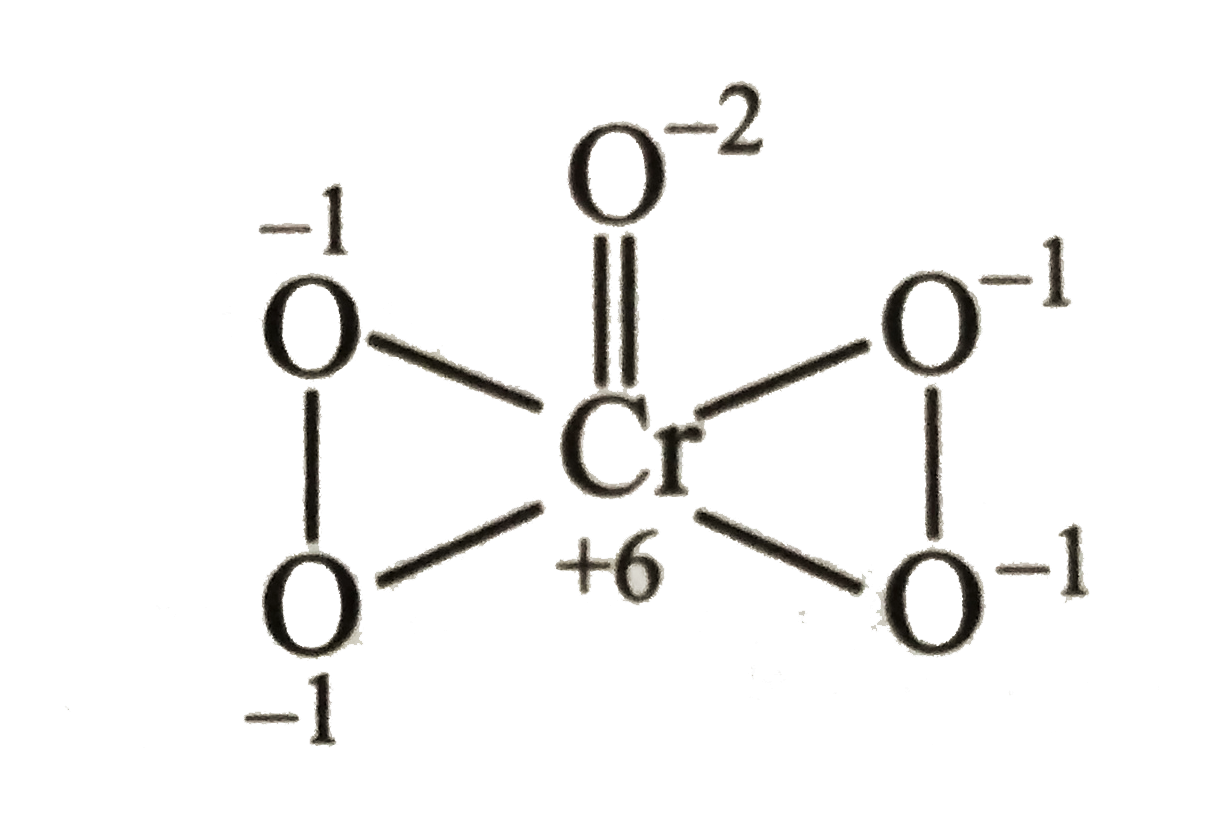
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REDOX REACTIONS
CENGAGE CHEMISTRY|Exercise Exercises (Single Correct)|91 VideosREDOX REACTIONS
CENGAGE CHEMISTRY|Exercise Exercises (Assertion-Reasoning)|15 VideosREDOX REACTIONS
CENGAGE CHEMISTRY|Exercise Exercises (Linked Comprehension)|24 VideosPURIFICATION OF ORGANIC COMPOUNDS AND QUALITATIVE AND QUANTITATIVE ANALYSIS
CENGAGE CHEMISTRY|Exercise Assertion Reasoning Type|5 VideosS-BLOCK GROUP 1 - ALKALI METALS
CENGAGE CHEMISTRY|Exercise Archives Subjective|8 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-REDOX REACTIONS-Exercises (Multiple Correct)
- Which of the following statements is//are correct?
Text Solution
|
- Which of the following statements about the following reaction is//are...
Text Solution
|
- The oxidation number of Cr is +6 in
Text Solution
|
- The oxidation number of carbon is zero in
Text Solution
|
- Which of the following has//have been arranged in order of decreasing...
Text Solution
|
- The oxidation number of carboxylic carbon atom in CH(3)COOH is
Text Solution
|
- Which of the following is//are autoredox reactions?
Text Solution
|
- Which of the following is// disproportionatin reactions?
Text Solution
|
- For the reaction KO(2)+H(2)O+CO(2)rarrKHCO(3)+O(2), the mechanism of r...
Text Solution
|
- Which of the following can be used both as an oxidant and a reductant?
Text Solution
|
- Which molecule represent by the bold atoms are in their highest oxidat...
Text Solution
|
- Which molecule represent by the bold atoms are in their lowest oxidati...
Text Solution
|
- Which of the following statements is//are correct about CH(2)=C Cl(2)
Text Solution
|
- Which of the following statemetns about tailing of Hg is//are correct?
Text Solution
|
- Which of the following is//are disproportionation redox changes?
Text Solution
|
- Which of the following statements about the reaction is//are correct? ...
Text Solution
|
- Which of the following substances undergo(s) disproportionation reacti...
Text Solution
|
- Which of the following represents redox reactions?
Text Solution
|
- Consider the redox reaction 2S(2)O(3)^(2-)+I(2)rarrS(4)O(6)^(2-)+2I^...
Text Solution
|
- Which of the following compounds acts both as an oxidising as wll as a...
Text Solution
|