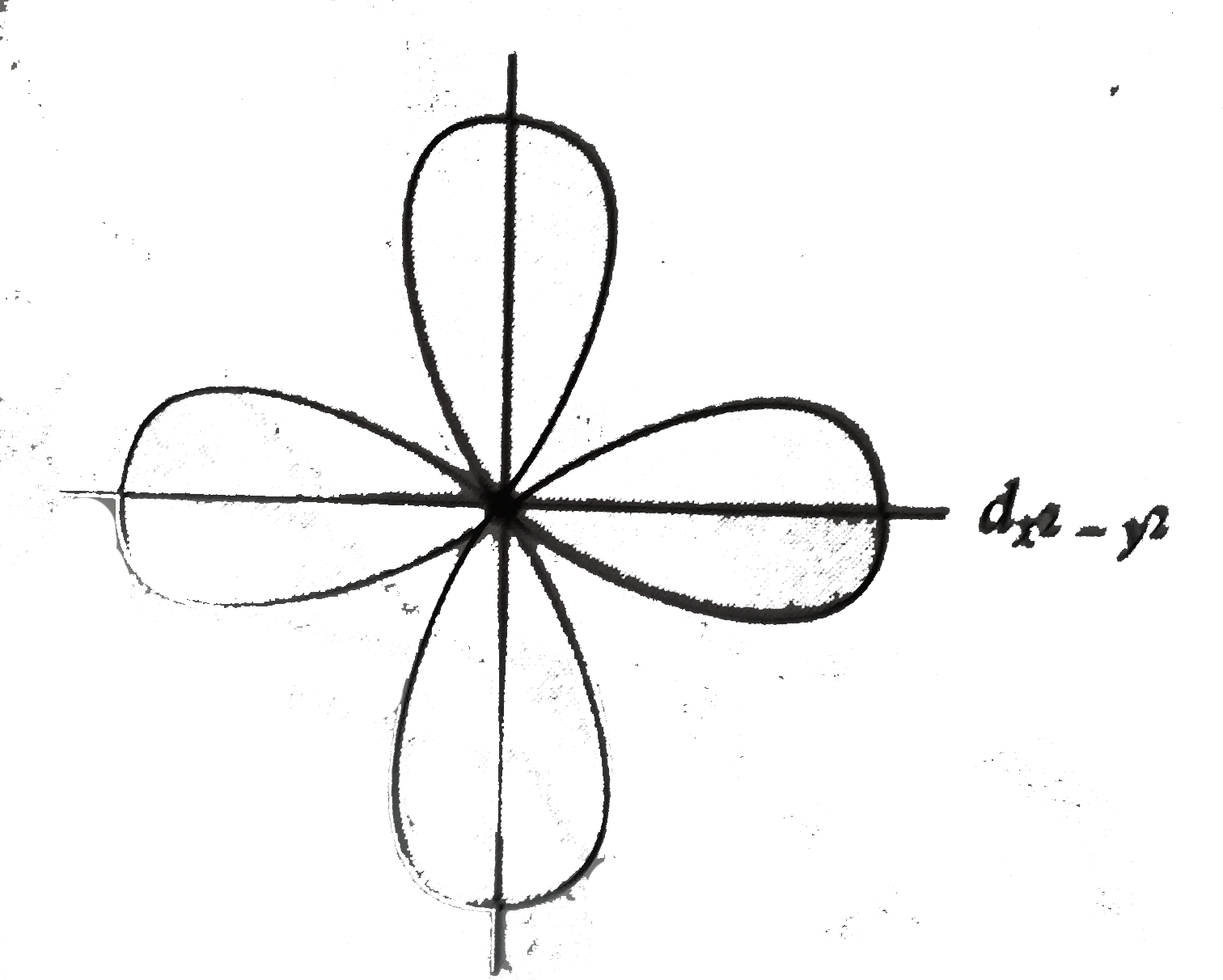Text Solution
Verified by Experts
Topper's Solved these Questions
ATOMIC STRUCTURE
CENGAGE CHEMISTRY|Exercise Archives Subjective|20 VideosATOMIC STRUCTURE
CENGAGE CHEMISTRY|Exercise Concept Applicationexercise (4.1)|11 VideosATOMIC STRUCTURE
CENGAGE CHEMISTRY|Exercise Archives Fill In The Balnks|8 VideosAPPENDIX - INORGANIC VOLUME 1
CENGAGE CHEMISTRY|Exercise chapter-7 Single correct answer|1 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY|Exercise Archives Subjective|15 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-ATOMIC STRUCTURE-Archives True And False
- The outer electronic configuration of the ground state chromium atom i...
Text Solution
|
- The energy of the elkectron in the 3d orbital is less than that in th...
Text Solution
|
- gamma rays are electromagnetic radiation of wavelength of 10^(-6)" to...
Text Solution
|
- The electron density in the xy- plane in 3d(x^(2) - y^(2) orbital is z...
Text Solution
|
- In a given electric field , the beta particle are deflected more than ...
Text Solution
|