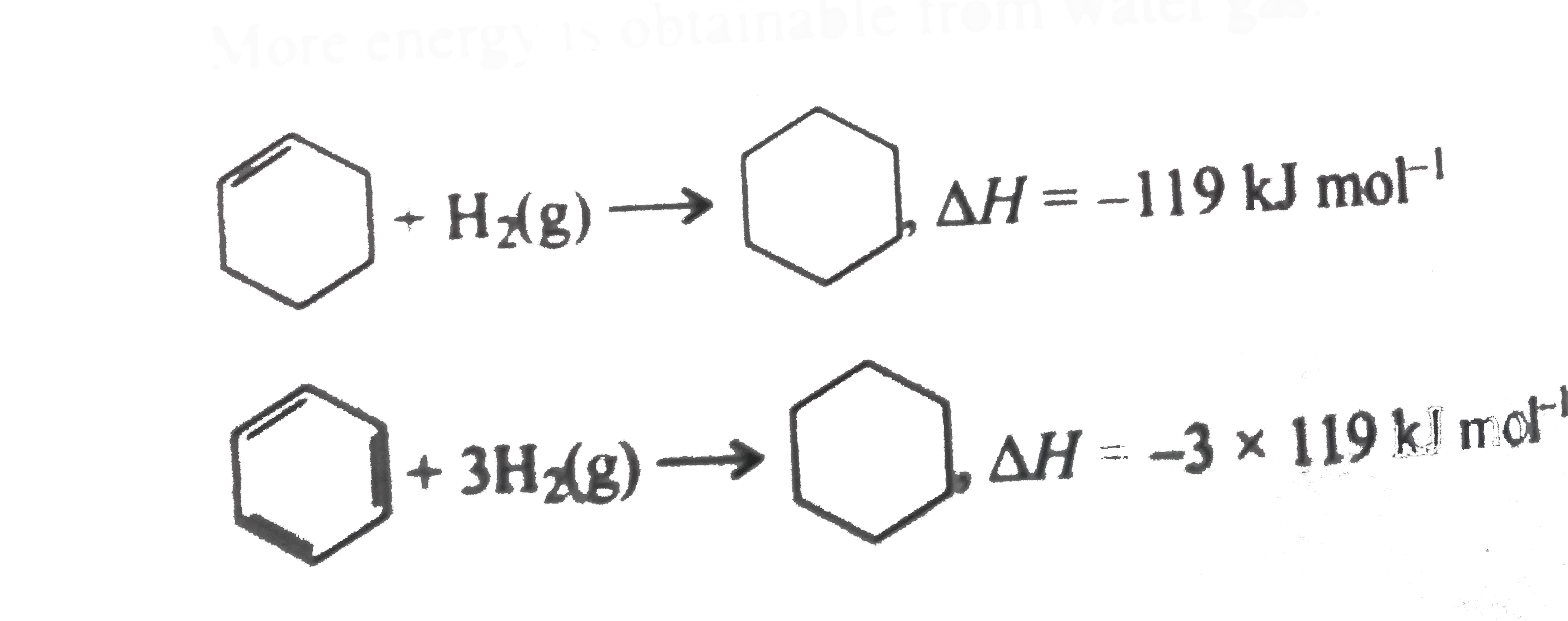
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-THERMODYNAMICS-Ex 6.3
- DeltaH combustion for CH(4),C(2)H(6), and C(3)H(8) are -210.8,-368.4 a...
Text Solution
|
- When 12.0g of C reacted with a limited quantity of oxygen, 57.5 kcal o...
Text Solution
|
- When 12.0g of C reacted with oxygen to form CO and CO(2) at 25^(@)C at...
Text Solution
|
- Given CaCI(2)(s) +aq rarr CaCI(2)(aq): DeltaH^(Theta) = 75 kJ mol^(-1)...
Text Solution
|
- The dissociation pressure of CaCO(3)(s) overset(Delta) rarr CaO(s) +...
Text Solution
|
- Calculate the enthalpy change for the following reaction: XeF(4) rar...
Text Solution
|
- The conversion of gaseous atoms K and F to K^(o+) and F^(ɵ) absorbs 0....
Text Solution
|
- While 1mol of ice melts at 0^(@)C and at constant pressure of 1atm, 14...
Text Solution
|
- At 25^(@)C, buring 0.2 "mole" H(2) with 0.1 mole O(2) to produce H(2)O...
Text Solution
|
- Calculate the enthalpy of formation of aniline. The enthalpy of combus...
Text Solution
|
- Calcualte the enthalpy change when infinitely dilute solution of CaCI(...
Text Solution
|
- The sublimation energy of a metal is 100kJmol^(-1) and its Ist and Iin...
Text Solution
|
- The C-H bond of the side chain in toluene, C(6)H(5)-CH(3), has a disso...
Text Solution
|
- Calculate Deltah for the eaction BaCO(3)(s)+2HCI(aq) rarr BaCI(2)(aq...
Text Solution
|
- Calculate the heat produced when 3.785 L of octabe reacts with oxygeb ...
Text Solution
|
- a. Cis-2-butene rarr trans-2-butane, DeltaH(1) b. Cis-2-butane rarr1...
Text Solution
|
- Calculate the proton affinity of NH(3)(g) from the following data (in ...
Text Solution
|
- In certan areas where coal is cheap, artificial gas is produced for ho...
Text Solution
|
- Calculate the enthalpy of combustion of benzene (l) on the basis of th...
Text Solution
|
- Find DeltaH of the process NaOH(s) rarr NaOH(g) Given: Delta(diss)...
Text Solution
|