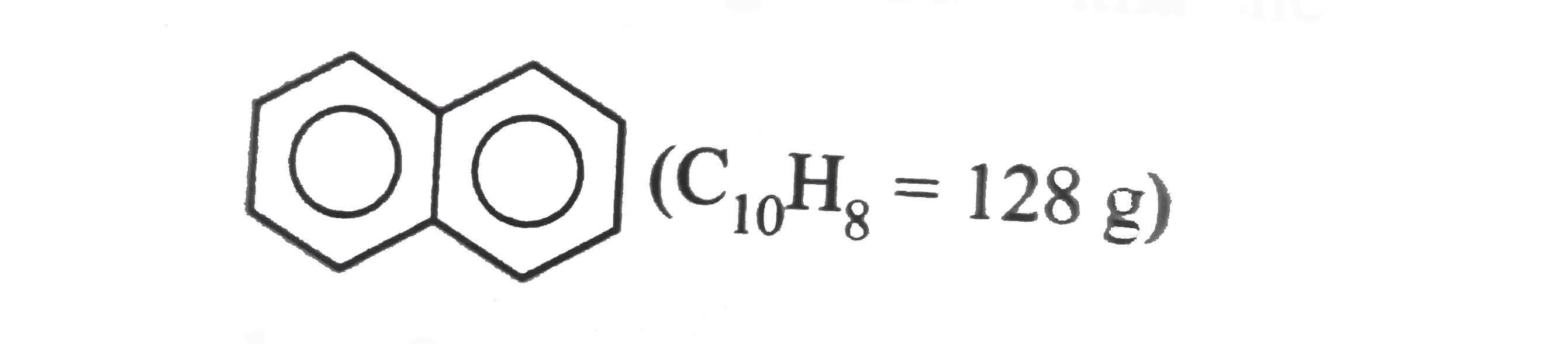Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-THERMODYNAMICS-Exercises (Subjective)
- If 2AI(s) +1(1)/(2) O(2)(g) rarr AI(2)O(3)(s), DeltaH =- 1667.8 kJ H...
Text Solution
|
- The thermochemical equation for solid and liquid rocket fuel are given...
Text Solution
|
- When 1g liquid naphthalene (C(10)H(8)) solidifies, 149J of heat is evo...
Text Solution
|
- a. A cylinder of gas is assumed to contain 11.2 kg of butane. If a nor...
Text Solution
|
- The free enegry changes for the two reactions given below are a. SO(...
Text Solution
|
- Given that: i. C("graphite") +O(2)(g) rarr CO(2)(g), DeltaH =- 393.7...
Text Solution
|
- Given: i. H(g) +CI(g) rarr HCI(g) DeltaH =- 431 kJ ii. HCI(g) +aq ...
Text Solution
|
- Given: NaCI(s) +aq rarr Na^(o+) (aq) +CI^(Theta) DeltaH = 3.9 kJ N...
Text Solution
|
- What would be the heat released when:
Text Solution
|
- Enthalpy of neutralisation of acetic acid by NaOh is -50.6 kJ mol^(-1)...
Text Solution
|
- The polymerisation of ethylene to linear polyethylene is represented b...
Text Solution
|
- An athelete is given 100g of glucose (C(6)H(12)O(6)) of energy equival...
Text Solution
|
- How much hea is liberated when one mole of gaseous Na^(o+) combines wi...
Text Solution
|
- State a chamical reaction in which DeltaH and DeltaU are equal .
Text Solution
|
- Which of the following is an intensive property. Surface tension, mass...
Text Solution
|
- State the first law of thermodynamics.
Text Solution
|
- For the reactions, N(2)(g) +3H(2)(g) rarr 2NH(3)(g) predict, whether t...
Text Solution
|
- Which has larger absolute entropy per mole? a. H(2)O(l) at 298 K or ...
Text Solution
|
- The dissolution of ammonium chloride in water is an endothermic proces...
Text Solution
|
- Does an aqueous solution of Mg^(2+) ions have larger entropy before or...
Text Solution
|