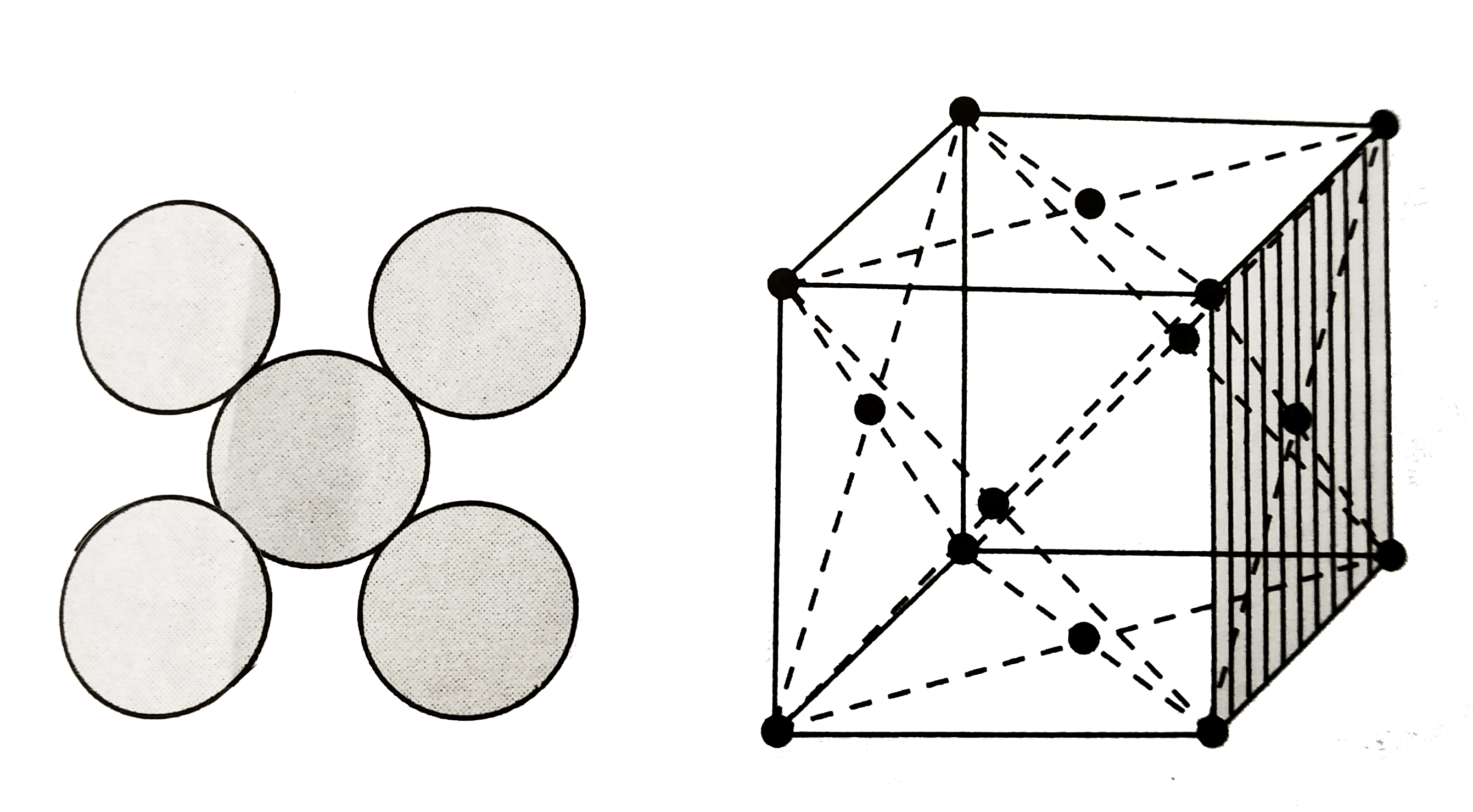
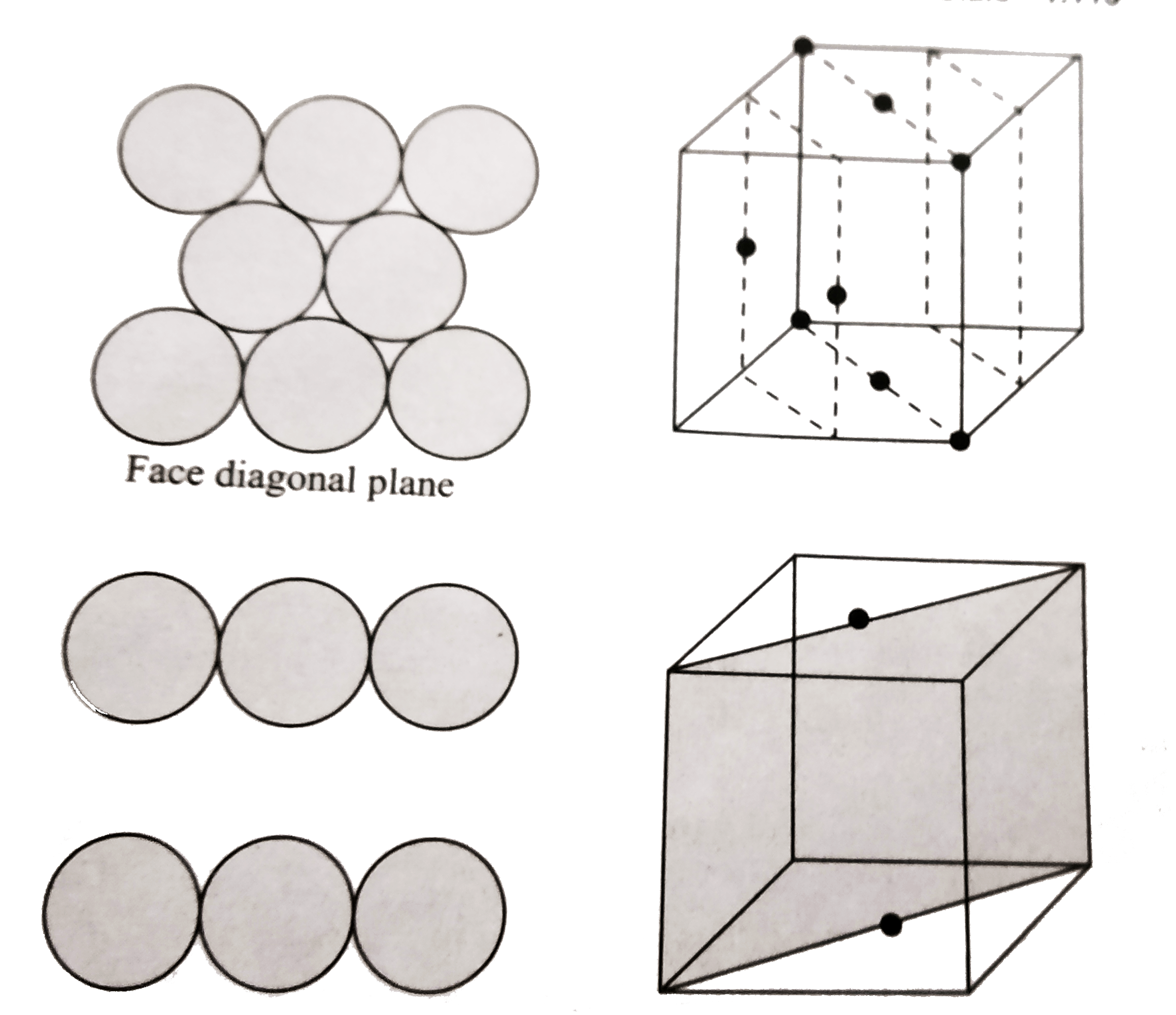
Text Solution
Verified by Experts
Topper's Solved these Questions
SOLID STATE
CENGAGE CHEMISTRY|Exercise Ex 1.1 (Subjective)|12 VideosSOLID STATE
CENGAGE CHEMISTRY|Exercise Ex 1.1 (Objective)|19 VideosSOLID STATE
CENGAGE CHEMISTRY|Exercise Exercises (Archives ) Interger|1 VideosREDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY|Exercise SUBJECTIVE TYPE|3 VideosSOLUTIONS
CENGAGE CHEMISTRY|Exercise Ex 2.3 (Objective)|9 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-SOLID STATE-Exercises (Archives ) Subjective
- Sodium metal crystallises in body centred cubic lattic with cell edge ...
Text Solution
|
- A metallic crystal cystallizes into a lattice containing a sequence of...
Text Solution
|
- Chromium metal crystallizes with a body-centred cubic lattice. The len...
Text Solution
|
- A metal crystallizes into two cubic phases, face-centred cubic and bod...
Text Solution
|
- The figure below show the locations of atoms in three crystallographic...
Text Solution
|
- You are given marbles of diameter 10mm. They are to be placed such tha...
Text Solution
|
- The crystal AB(rock salt structure) has molecular weight 6.023Y u, whe...
Text Solution
|
- An element crystallises in f.c.c. lattice having edge length 400 p m. ...
Text Solution
|
- The edge length of unit cell of a metal having molecular weight 75 g m...
Text Solution
|