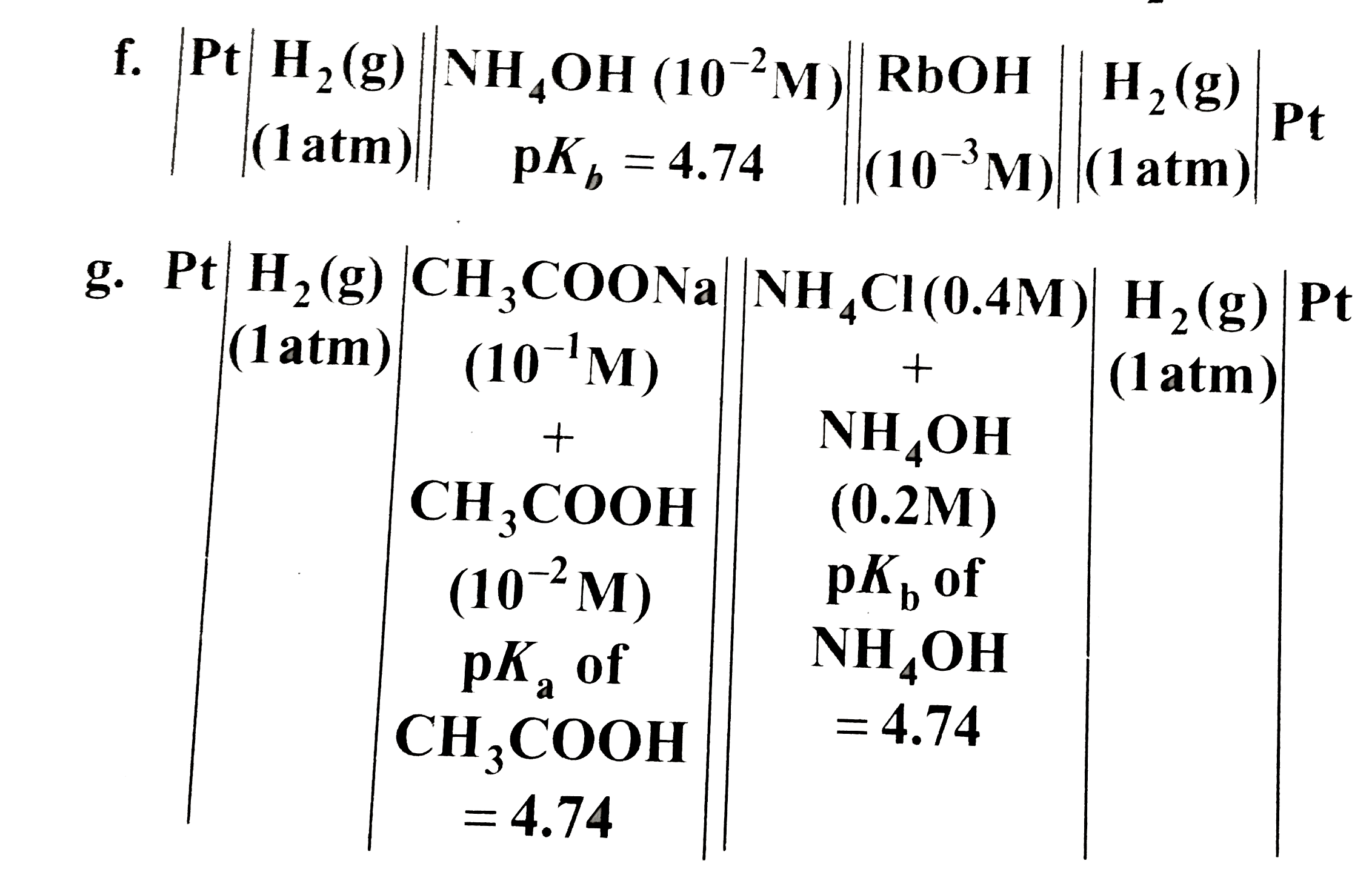Text Solution
Verified by Experts
Topper's Solved these Questions
ELECTROCHEMISTRY
CENGAGE CHEMISTRY|Exercise Solved Examples (Electrochemical Cell)|36 VideosELECTROCHEMISTRY
CENGAGE CHEMISTRY|Exercise Solved Examples(Electrolysis And Electrolytic Cells)|12 VideosD AND F BLOCK ELEMENTS
CENGAGE CHEMISTRY|Exercise Archives Subjective|29 VideosGENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTS
CENGAGE CHEMISTRY|Exercise Archives (Subjective)|14 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-ELECTROCHEMISTRY-Archieves Subjective
- Calculate the EMF of the following concentration cells a 30^(@)C and p...
Text Solution
|
- A current of 3.7 A is passed for 6hrs. Between Ni electrodes in 0.5 L ...
Text Solution
|
- Consider the cell : Zn|Zn^(2+)(aq)(1.0M)||Cu^(2+)(aq)(1.0M)||Cu Th...
Text Solution
|
- In an electrolysis experiment, current was passed for 5h through two c...
Text Solution
|
- How long a current of 3A has to be passed through a solution of silver...
Text Solution
|
- Give reasons in one or two sentences :" anhydrous HCl is a bad conduc...
Text Solution
|
- The EMF of the following cellis 1.05V at 25^(@)C: Pt,H(2)(g)(1.0 atm...
Text Solution
|
- During the discharge of a lead storage battery, the density of sulphur...
Text Solution
|
- A 100-W,100-V incardescent lamp is connected in series with an electro...
Text Solution
|
- A cell contains two hydrogen electrodes. The negative electrode is in ...
Text Solution
|
- In a fuel cell, hydrogen and oxygen react to produce electricity. In p...
Text Solution
|
- An acidic solution of Cu^(2+0 salt containing 0.4g of Cu^(2+) is elect...
Text Solution
|
- The standard reduction potential at 25^(@)C of the reaction 2H(2)O+2...
Text Solution
|
- The standard reduction potential of Cu^(2+)|Cu and Ag^(o+)|Ag electrod...
Text Solution
|
- Calculate the quantity of electricity that would be required to reduce...
Text Solution
|
- Zinc granules are added in excess to 500 mL of 1M Ni(NO(3))(2) solutio...
Text Solution
|
- A current of 1.70A is passed trhough 300.0 mL of 0.160M solution of Zn...
Text Solution
|
- For the galvanic cell: Ag|AgCI(s)|KCI(0.2M)||KBr(0.001M)|AgBr(s)|Ag, c...
Text Solution
|
- An aqueous solution of NaCl on electrolysis gives H(2)(g), Cl(2)(g), a...
Text Solution
|
- The standard reduction potential for the half cell : NO(3)^(-)(aq)+2...
Text Solution
|
- Chromium metal can be plated out from an acidic solution containing Cr...
Text Solution
|