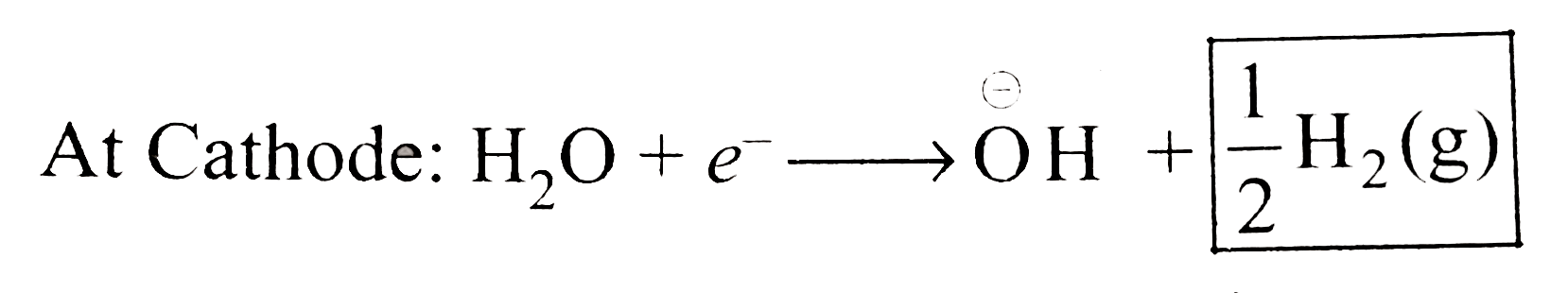
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
CENGAGE CHEMISTRY|Exercise Ex 3.3 (Objective)|10 VideosELECTROCHEMISTRY
CENGAGE CHEMISTRY|Exercise Exercise(Linked Comprehension )|30 VideosELECTROCHEMISTRY
CENGAGE CHEMISTRY|Exercise Ex 3.1 (Objective)|28 VideosD AND F BLOCK ELEMENTS
CENGAGE CHEMISTRY|Exercise Archives Subjective|29 VideosGENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTS
CENGAGE CHEMISTRY|Exercise Archives (Subjective)|14 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-ELECTROCHEMISTRY-Ex 3.2 (Objective)
- The number of moles of Zn^(2+) ions deposited when a current of 1.5A i...
Text Solution
|
- Molten NaCl is electrolyzed in a cell called
Text Solution
|
- A dilute aqueous solution of sodium fluoride is electrolyzed, the prod...
Text Solution
|
- If 0.224 L of H(2)(g) is formed at the cathode of one cell at STP, how...
Text Solution
|
- A certain amount of charge is passed through acidulated water. A total...
Text Solution
|
- 1L of 1M CuSO(4) solution is electrolyzed using Pt cathode and Cu anod...
Text Solution
|
- In a Ni-Cd battery ( more than one correct )
Text Solution
|
- Rusting of iron is
Text Solution
|
- In H(2)-O(2) fuel cell, the reaction occurring at cathode is
Text Solution
|
- The cathode reaction during the charging of a lead - acid battery lead...
Text Solution
|
- Which of the following cells is rechargeable ?
Text Solution
|
- During discharging of alead storage battery
Text Solution
|
- Explain how rusting of iron is envisaged as setting up of an electroCH...
Text Solution
|
- How many Faradays are required to reduce 1 mol of BrO(3)^(c-) to Br^(c...
Text Solution
|
- Which of the following aqueous solutions remains neutral after electro...
Text Solution
|
- Same quantity of current is passed through molten NaCl and molten cryo...
Text Solution
|
- In the electrolysis of a 40L CuSO(4) solution, there are two possible ...
Text Solution
|
- Two platinum electrodes were immersed in a solution of CuSO(4) and ele...
Text Solution
|
- Aluminium oxide may be electorlysed at 1000^@C to furnish aluminim met...
Text Solution
|
- The density of copper is 8.95 g mL^(-1). Find out the number of coulom...
Text Solution
|