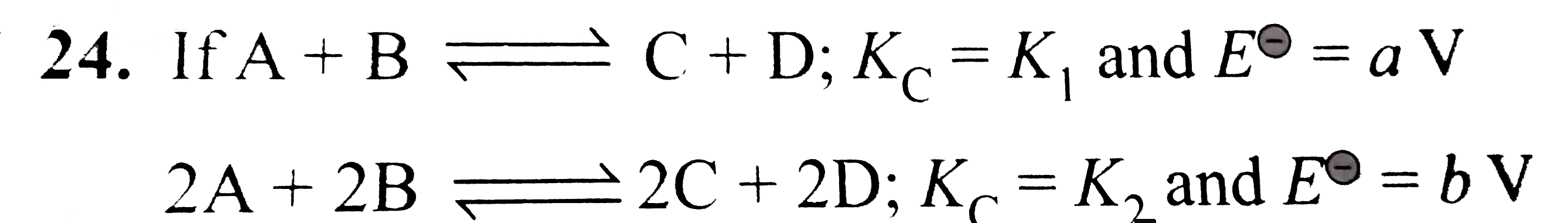A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
CENGAGE CHEMISTRY|Exercise Exercises Ingle Correct|178 VideosELECTROCHEMISTRY
CENGAGE CHEMISTRY|Exercise Exerciseassertion -Reasoning|25 VideosELECTROCHEMISTRY
CENGAGE CHEMISTRY|Exercise Exercise(Linked Comprehension )|30 VideosD AND F BLOCK ELEMENTS
CENGAGE CHEMISTRY|Exercise Archives Subjective|29 VideosGENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTS
CENGAGE CHEMISTRY|Exercise Archives (Subjective)|14 Videos
CENGAGE CHEMISTRY-ELECTROCHEMISTRY-Exercisemultiple Correct Ansers
- In the atmosphere of industrial smog, copper corrodes to form
Text Solution
|
- The tarnishing of silver ornaments in atmosphere is due to
Text Solution
|
- If then
Text Solution
|
- Rusting of iron is catalyzed by
Text Solution
|
- Select the wrong relation (s).
Text Solution
|
- Select the correct statements (s) about NHE.
Text Solution
|
- In which of the following salt bridge is not needed ?
Text Solution
|
- Select the correct statements if 9.65 A current is passed for 1 hour t...
Text Solution
|
- The temperature coefficient of the cell is ((delE)/(delT))(P). Choose ...
Text Solution
|
- During discharging of alead storage battery
Text Solution
|
- Which of the following statements is // are correct ?
Text Solution
|
- Identify the correct statements (s):
Text Solution
|
- Which of the following cells is // are rechargeable or secondary cell ...
Text Solution
|
- For I(2)+2e^(-) rarr 2I^(c-), standard reduction potential =+0.54 V. F...
Text Solution
|
- Consider the cell : Pt|H(2)(p(1)atm)|H^(o+)(x(1)M) || H^(o+)(x(2)M)|...
Text Solution
|
- Which of the following changes will cause the free energy of a cell re...
Text Solution
|
- During the working of a galvanic cell and with the passage of time.
Text Solution
|
- In the following electrochemical cell : Zn|Zn^(2+)||H^(o+)|(H(2))Pt ...
Text Solution
|
- Consider the cell : Cd(s)|Cd^(2+)(1.0M)||Cu^(2+)(1.0M)|Cu(s) If we...
Text Solution
|
- Electrolysis of aqueous solutions of which of the following substance...
Text Solution
|