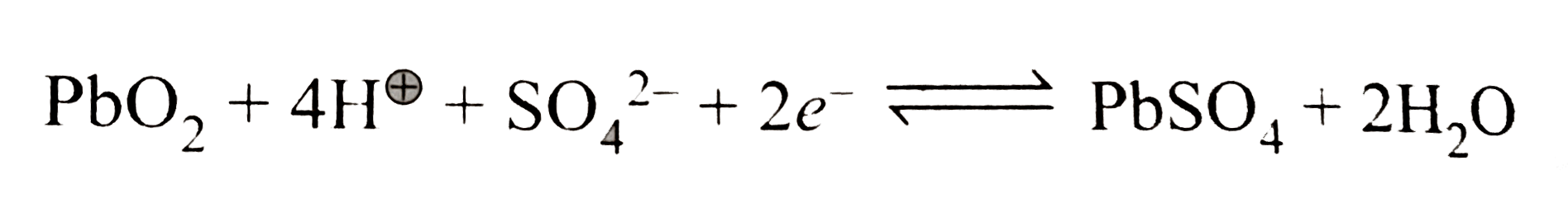Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
CENGAGE CHEMISTRY|Exercise Exercisefill In The Blanks|25 VideosELECTROCHEMISTRY
CENGAGE CHEMISTRY|Exercise Exercisetrue / False|40 VideosELECTROCHEMISTRY
CENGAGE CHEMISTRY|Exercise Exerciseassertion -Reasoning|25 VideosD AND F BLOCK ELEMENTS
CENGAGE CHEMISTRY|Exercise Archives Subjective|29 VideosGENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTS
CENGAGE CHEMISTRY|Exercise Archives (Subjective)|14 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-ELECTROCHEMISTRY-Exerciseinterger
- During the electrolysis of conc H(2)SO(4), it was found that H(2)S(2)...
Text Solution
|
- How many Faradays are required to reduce 1 mol of BrO(3)^(c-) to Br^(c...
Text Solution
|
- The total number of Faradays required to oxidize the following separat...
Text Solution
|
- For the oxidation of ferric oxalate to CO(2), 18F of electricity is re...
Text Solution
|
- During the discharge of a lead storage battery, the density of 40% H(2...
Text Solution
|
- In Question above, the number of ampere hours for which the battery is...
Text Solution
|
- DeltaG for the reaction : (4)/(3) Al+O(2)rarr (2)/(3)Al(2)O(3) is...
Text Solution
|
- When electrolysis of KCl is doen in alkaline medium, 10g of KClO(3) is...
Text Solution
|