Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
CENGAGE CHEMISTRY|Exercise Archieves (Linked Comprehension )|13 VideosELECTROCHEMISTRY
CENGAGE CHEMISTRY|Exercise Archieves Multiple Correct Ansers|2 VideosELECTROCHEMISTRY
CENGAGE CHEMISTRY|Exercise Exercisefill In The Blanks|25 VideosD AND F BLOCK ELEMENTS
CENGAGE CHEMISTRY|Exercise Archives Subjective|29 VideosGENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTS
CENGAGE CHEMISTRY|Exercise Archives (Subjective)|14 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-ELECTROCHEMISTRY-Exercisetrue / False
- In Castner - Kellner cell for the manufacture of NaOH( caustic soda ),...
Text Solution
|
- The amount of charge carried by an electrone is the same as carried by...
Text Solution
|
- The same quantity of electricity is passed through Al(2)(SO(4))(3) and...
Text Solution
|
- Metals always liberate H(2)(g) from acids.
Text Solution
|
- Sn^(2+) and Fe^(3+) cannot exist in the same solution.
Text Solution
|
- The addition of a crystal of I(2) to NaBr turns the solution violet.
Text Solution
|
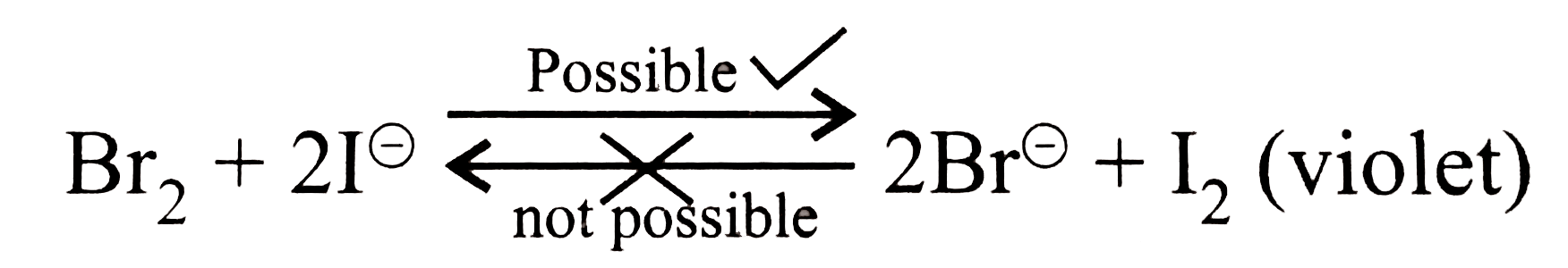
- The addition of Br(2) to NaI turns the solution violet.
Text Solution
|
- Lead storage battery has anode and cathode made up of Pb.
Text Solution
|
- Electrode potential for the electrode M^(n+)|M with concerntration is ...
Text Solution
|
- In the electroplating of silver,AgNO(3) solution is usually used as an...
Text Solution
|
- The conductance of electrolyte solution increases wih temperature.
Text Solution
|
- Resistivity is reciprocal of molar conductivity of electrolyte.
Text Solution
|
- Cell constant has unit m^(-1).
Text Solution
|
- The conductivity of molten KCl is due to the movement of K^(o+) and Cl...
Text Solution
|
- Solid KCl is a good conductor of electricity.
Text Solution
|
- Molten Na(2)SO(4) is a good conductor because of mobile electrons.
Text Solution
|
- Cathode is negative terminal both in electrochemical and electrolytic ...
Text Solution
|
- Reduction occurs at cathdooe both in galvanic as well as in electrolyt...
Text Solution
|
- The chemical change in an electrolytic cell is non- spontaneous.
Text Solution
|
- The cell voltage is independent of the size of the cell or electrodes.
Text Solution
|