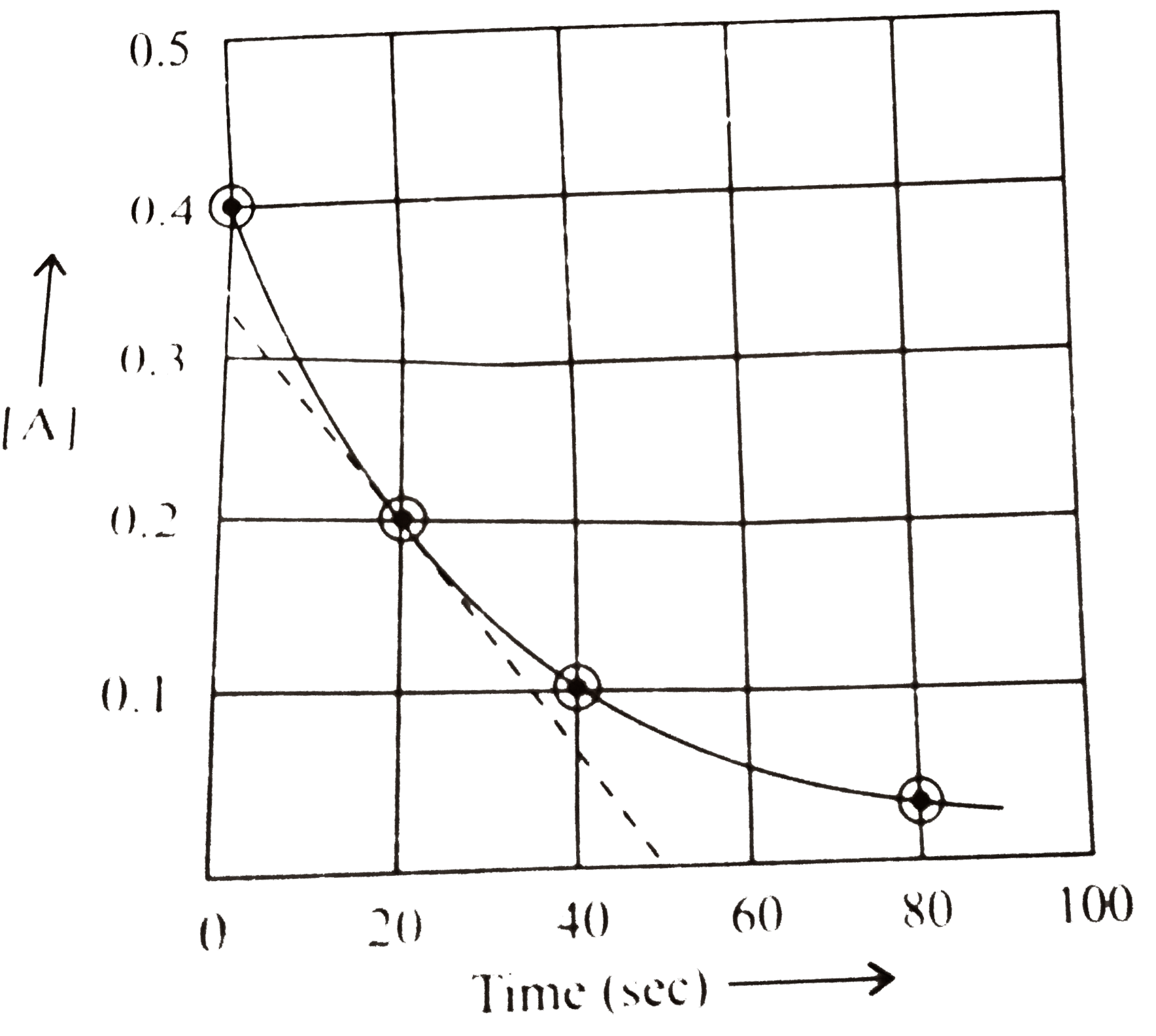
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
CENGAGE CHEMISTRY|Exercise Exercises Single Correct|177 VideosCHEMICAL KINETICS
CENGAGE CHEMISTRY|Exercise Exercises Assertion-Reasoning|22 VideosCHEMICAL KINETICS
CENGAGE CHEMISTRY|Exercise Exercises Linked Comprehension|59 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
CENGAGE CHEMISTRY|Exercise Exercises Archives (Analytical And Descriptive)|34 VideosCOORDINATION COMPOUNDS
CENGAGE CHEMISTRY|Exercise Archives Subjective|18 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-CHEMICAL KINETICS-Exercises Multiple Correct
- Which of the following plots are correctly made for the reaction nA hA...
Text Solution
|
- Rate constant k varies with temperature by equation log k (min^(-1)) =...
Text Solution
|
- Which of the following reaction (s) is // are of the first order ?
Text Solution
|
- The baiss theory behind Arrhenius' equation is that
Text Solution
|
- In Arrhenius equation k = A exp (-(E(a))/(RT)). A may be termed as the...
Text Solution
|
- Select the correct statement (s).
Text Solution
|
- For a gaseous reaction: A(g) rarr B(g), the rate expresison may be giv...
Text Solution
|
- The rate of formation of C(6)H(6)+3H(2) underset(k(b))overset(k(f))hAr...
Text Solution
|
- A certain reaction A rarr B follows the given concentration (Molarity)...
Text Solution
|
- For a first order reaction,
Text Solution
|
- Which of the following statement (s) is/are correct
Text Solution
|
- Which of the following statements is/are correct?
Text Solution
|
- The distribution of molecular kinetic energy at two temperature is as ...
Text Solution
|
- Which of the following isomerization reactions is/are of the first ord...
Text Solution
|
- Which of the following is/are examples of unimolecular reactions?
Text Solution
|
- Which of the followinf is/are correct?
Text Solution
|
- Zn+2H^(o+)rarrZn^(2+)+H(2) The half-life periof is independent of th...
Text Solution
|
- Which of the following is/are examples of pseudo unimolecular reaction...
Text Solution
|
- In which of the following ways does an activated complex differ form a...
Text Solution
|
- Which of the following statements is/are correct?
Text Solution
|