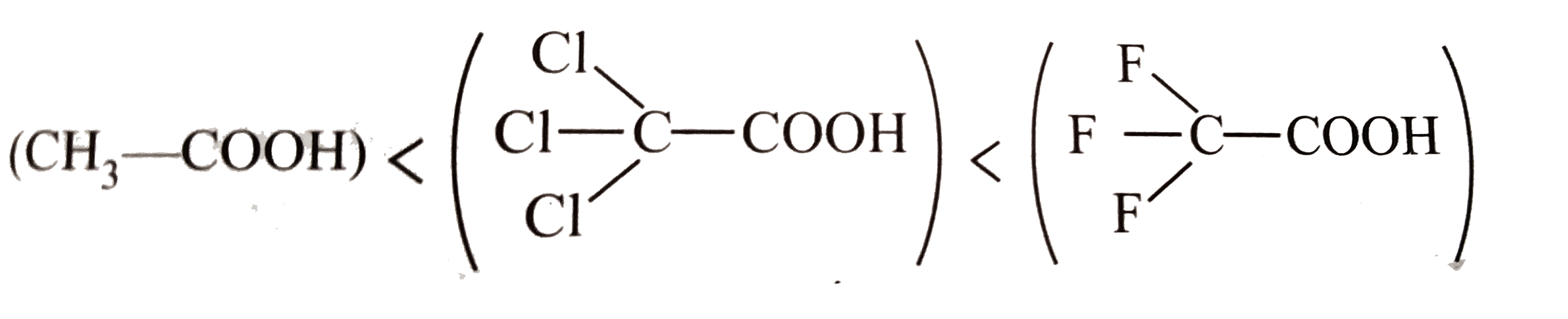Text Solution
Verified by Experts
Topper's Solved these Questions
NCERT BASED EXERCISE
CENGAGE CHEMISTRY|Exercise Short Answer Type Questions(ElectroCHMmical Cell)|4 VideosNCERT BASED EXERCISE
CENGAGE CHEMISTRY|Exercise Short Answer Type Questions(Electrochmical Cell)|12 VideosNCERT BASED EXERCISE
CENGAGE CHEMISTRY|Exercise Short Answer Type Questions|69 VideosGRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTS
CENGAGE CHEMISTRY|Exercise Exercises Archives (Linked Comprehension)|1 VideosNUCLEAR CHEMISTRY
CENGAGE CHEMISTRY|Exercise Archives Subjective|13 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-NCERT BASED EXERCISE-NCERT Exercise
- Nalorphene (C(19)H(22)NO(3)), similar to morphine , is used to combat ...
Text Solution
|
- Calculate the amound of benzoic acid (C(6)H(5)COOH) required for prepa...
Text Solution
|
- The depression in freeqing point of water observed for the same amount...
Text Solution
|
- Calculate the depression in the freezing point of water when 10g of CH...
Text Solution
|
- 19.5g of CHM(2)FCOOH is dissolved in 500g of water . The depression i...
Text Solution
|
- The vapour pressure of water at 293K is 17.535mm Hg. Calculate the va...
Text Solution
|
- Henry's law constant for the molality of methane in benzene at 298K is...
Text Solution
|
- 100g of liquid A( molar mass 140 g mol ^(-1)) was dissolved in 1000g ...
Text Solution
|
- Benzene and toluene form ideal solution over the entire range of comp...
Text Solution
|
- The air is a mixture of a number of gases. The major components are ox...
Text Solution
|
- Determine the amount of CaCl(2) (i = 2.47) dissolved in 2.5 L of water...
Text Solution
|
- Determine the osmotic pressure of a solution prepared by dissolving 25...
Text Solution
|
- Arrange the following metals in the order in whiCHM they displace eaCH...
Text Solution
|
- Given standard electrode potentials K^(o+)|K=-2.93V, Ag^(o+)|Ag=0.80...
Text Solution
|
- Depict the galvanic in whiCHM the reaction : Zn(s)+2Ag^(o+)(aq) rarr...
Text Solution
|
- Calculate the standard cell potentials of galvanic cell in whiCHM the...
Text Solution
|
- Write the Nernst equation and EMF of the following cells at 298K : a...
Text Solution
|
- In the button cells widely used in watCHMes and other devices the foll...
Text Solution
|
- Define conductivity and molar conductivity for the solution of an ele...
Text Solution
|
- The conductivity of 0.20M solution of KCl at 298K is 0.0248 S cm^(-1) ...
Text Solution
|