Text Solution
Verified by Experts
Topper's Solved these Questions
CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY|Exercise Concept Application Type|7 VideosCLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY|Exercise Linked Comprehesion Type|12 VideosCLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY|Exercise Solved Example|5 VideosCHEMICAL EQUILIBRIUM
CENGAGE CHEMISTRY|Exercise Archives (Subjective)|11 VideosGENERAL ORGANIC CHEMISTRY
CENGAGE CHEMISTRY|Exercise Analytical and Descriptive|1 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS-Exercises
- write the structural formula for each of the following a. A 3^(@) am...
Text Solution
|
- Indicate the following as 1^(@), 2^(@), and 3^(@)
Text Solution
|
- Indicate the following as 1^(@), 2^(@), and 3^(@)
Text Solution
|
- Write the structural formula for seven compounds with the formula C(3)...
Text Solution
|
- There are seven isomeric compounds with the formula C(4) H(10) O Write...
Text Solution
|
- There are four alky1 chlorides with the formula C(4) H(9) CI. Writre t...
Text Solution
|
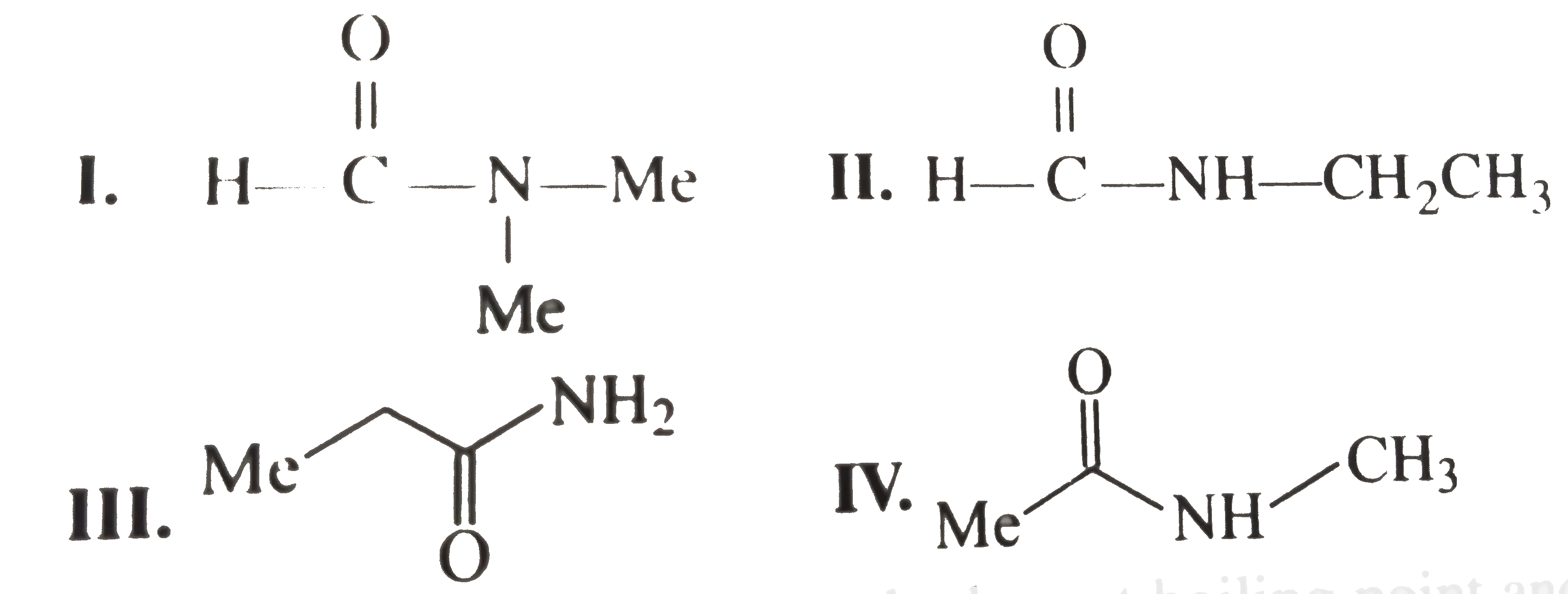
- There are four amides with the formula C(3) H(7) NO. Write their str...
Text Solution
|
- Write the IUPAC name of the compound (A) which is a 2-methy1 branched ...
Text Solution
|
- Write the IUPAC name of the compound (A) which is a 2-methyl alkane wi...
Text Solution
|
- Write the IUPAC name of the compound (A) in which the molar rations of...
Text Solution
|