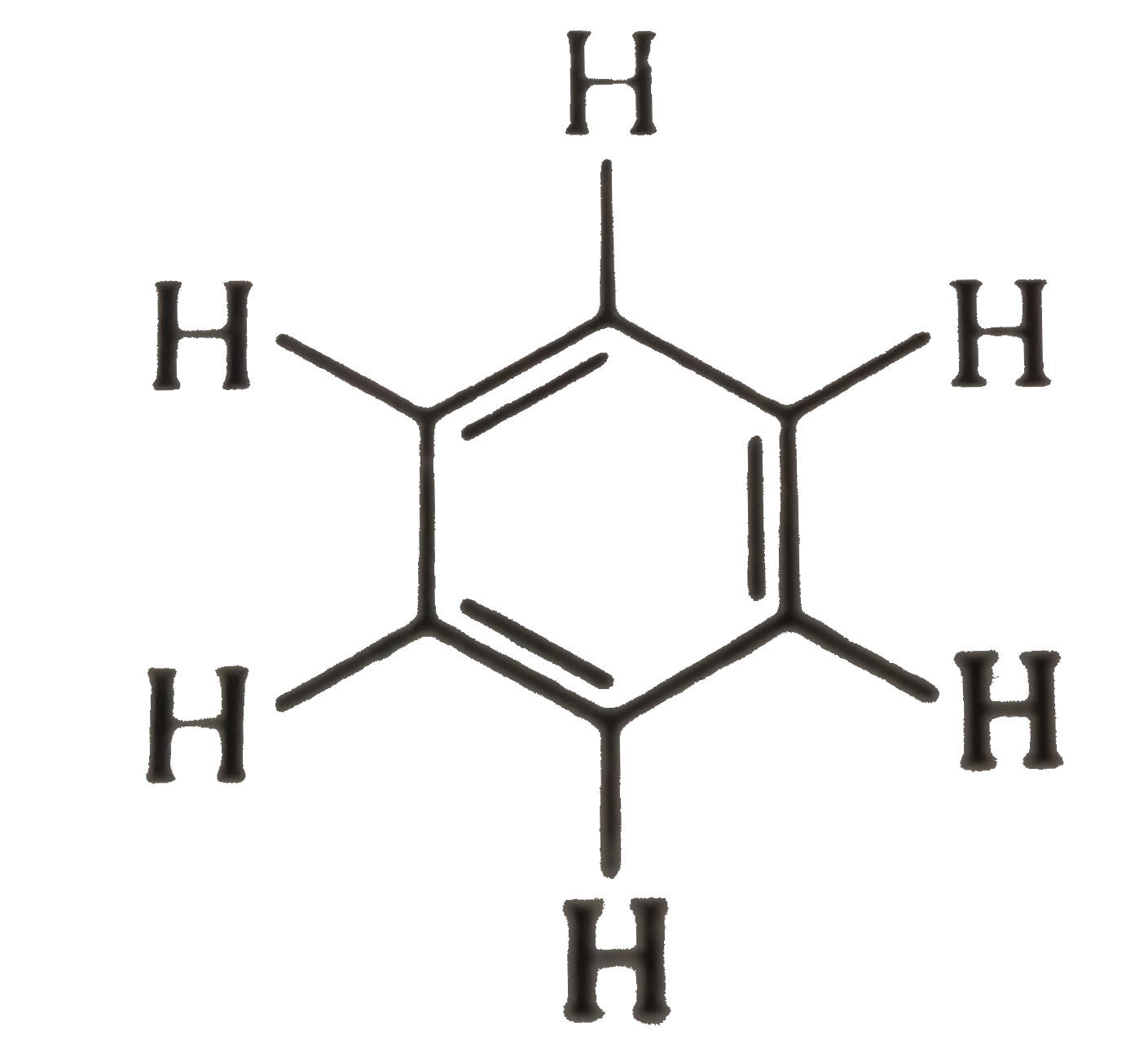
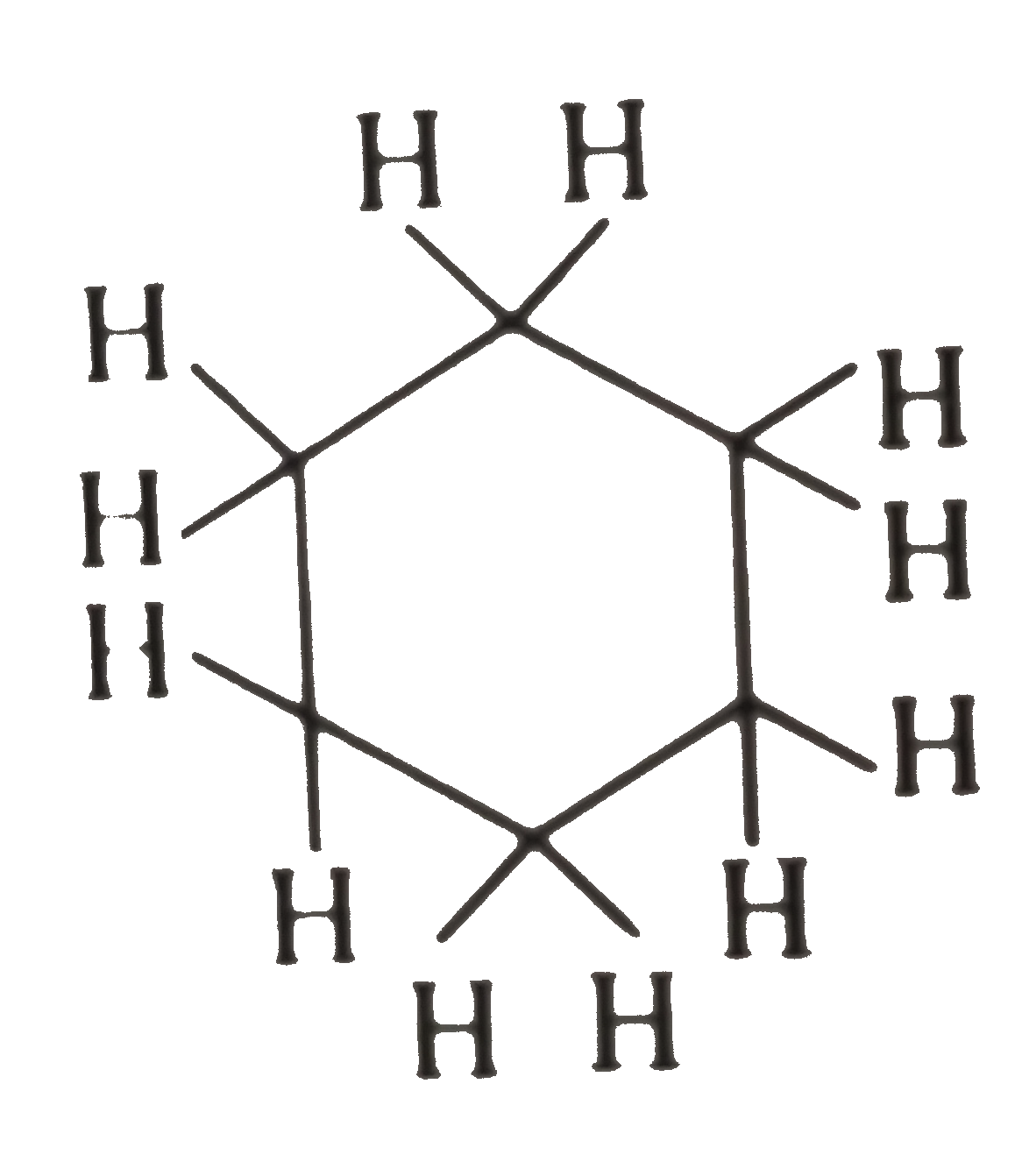
Text Solution
Verified by Experts
Topper's Solved these Questions
CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY|Exercise Linked Comprehesion Type|12 VideosCLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY|Exercise Multiple corrcct Answers Types|11 VideosCLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY|Exercise Exercises|10 VideosCHEMICAL EQUILIBRIUM
CENGAGE CHEMISTRY|Exercise Archives (Subjective)|11 VideosGENERAL ORGANIC CHEMISTRY
CENGAGE CHEMISTRY|Exercise Analytical and Descriptive|1 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS-Concept Application Type
- Given the hybridistaiton state of each carbon in the following compoun...
Text Solution
|
- Indicate the sigma- and pi - bonds in the following molecules: I. C(...
Text Solution
|
- Write the bond line formula for the following compounds: I. IIsoprop...
Text Solution
|
- Give the IUPAC names of the following compounds a. b. c.
Text Solution
|
- Which of the following represents the corrcet IUPAC name for the compo...
Text Solution
|
- Draw the formulae for the first five numbers of each homologous series...
Text Solution
|
- Identify the functional groups in the following compounds.
Text Solution
|