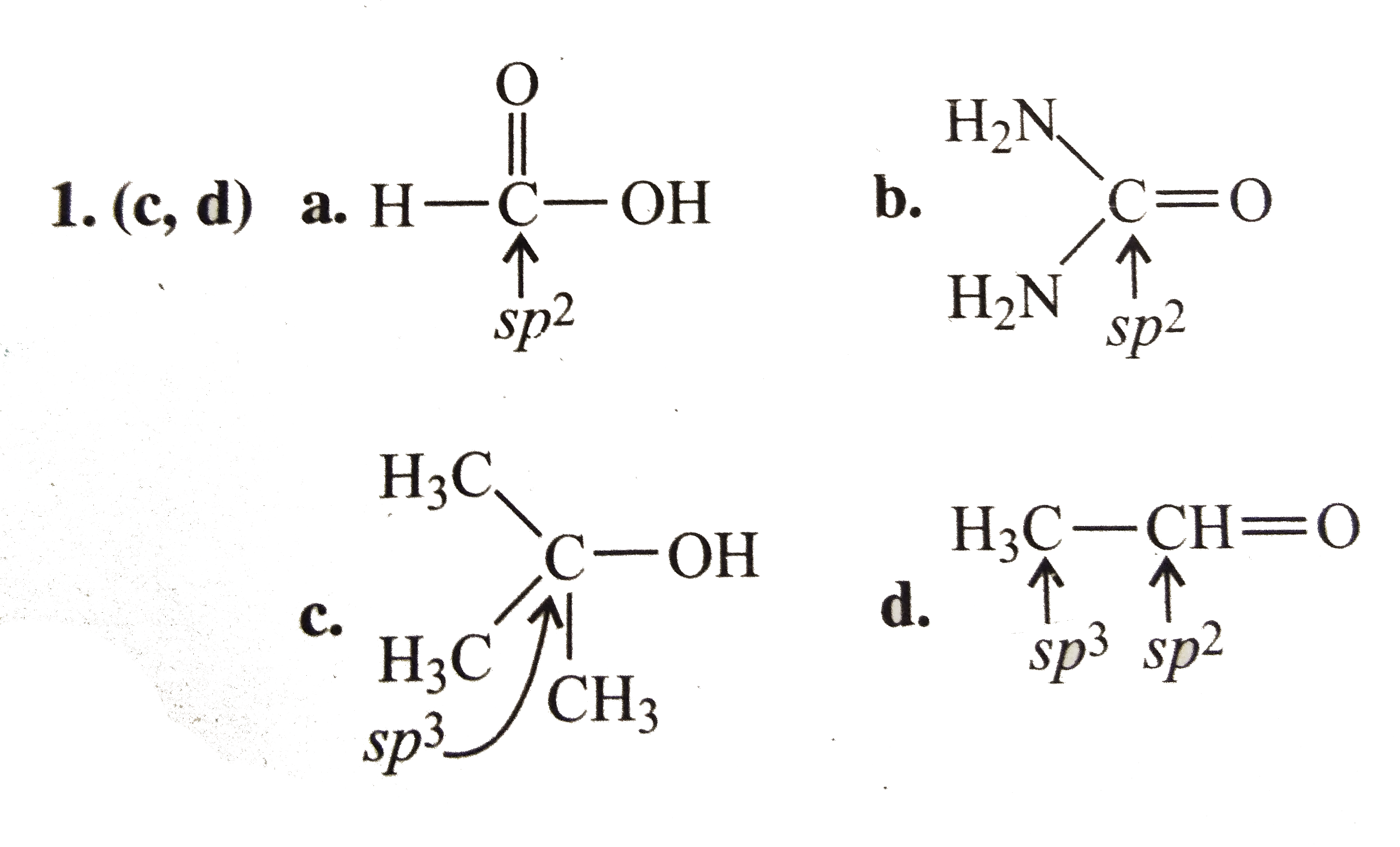
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY|Exercise Single correct Answer Type|44 VideosCLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY|Exercise Assertion-Reasoning Type|5 VideosCLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY|Exercise Linked Comprehesion Type|12 VideosCHEMICAL EQUILIBRIUM
CENGAGE CHEMISTRY|Exercise Archives (Subjective)|11 VideosGENERAL ORGANIC CHEMISTRY
CENGAGE CHEMISTRY|Exercise Analytical and Descriptive|1 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS-Multiple corrcct Answers Types
- Which of the following statements is//are wrongs?
Text Solution
|
- Which of the following statements is//are correct?
Text Solution
|
- Which of the following statements is/are wrong?
Text Solution
|
- Which of the following statements is//are correct?
Text Solution
|
- Which of the following statements is//are correct?
Text Solution
|
- Which of the following statements is//are correct? (i)The IUPAC name o...
Text Solution
|
- Which of the following statements is//are correct?
Text Solution
|
- Which of the following statements is//are correct?
Text Solution
|
- Which of the following statements is//are correct?
Text Solution
|
- Which of the following statements is//are correct?
Text Solution
|
- The compounds in which C uses its sp^(3)- hybrid orbitals for bond for...
Text Solution
|