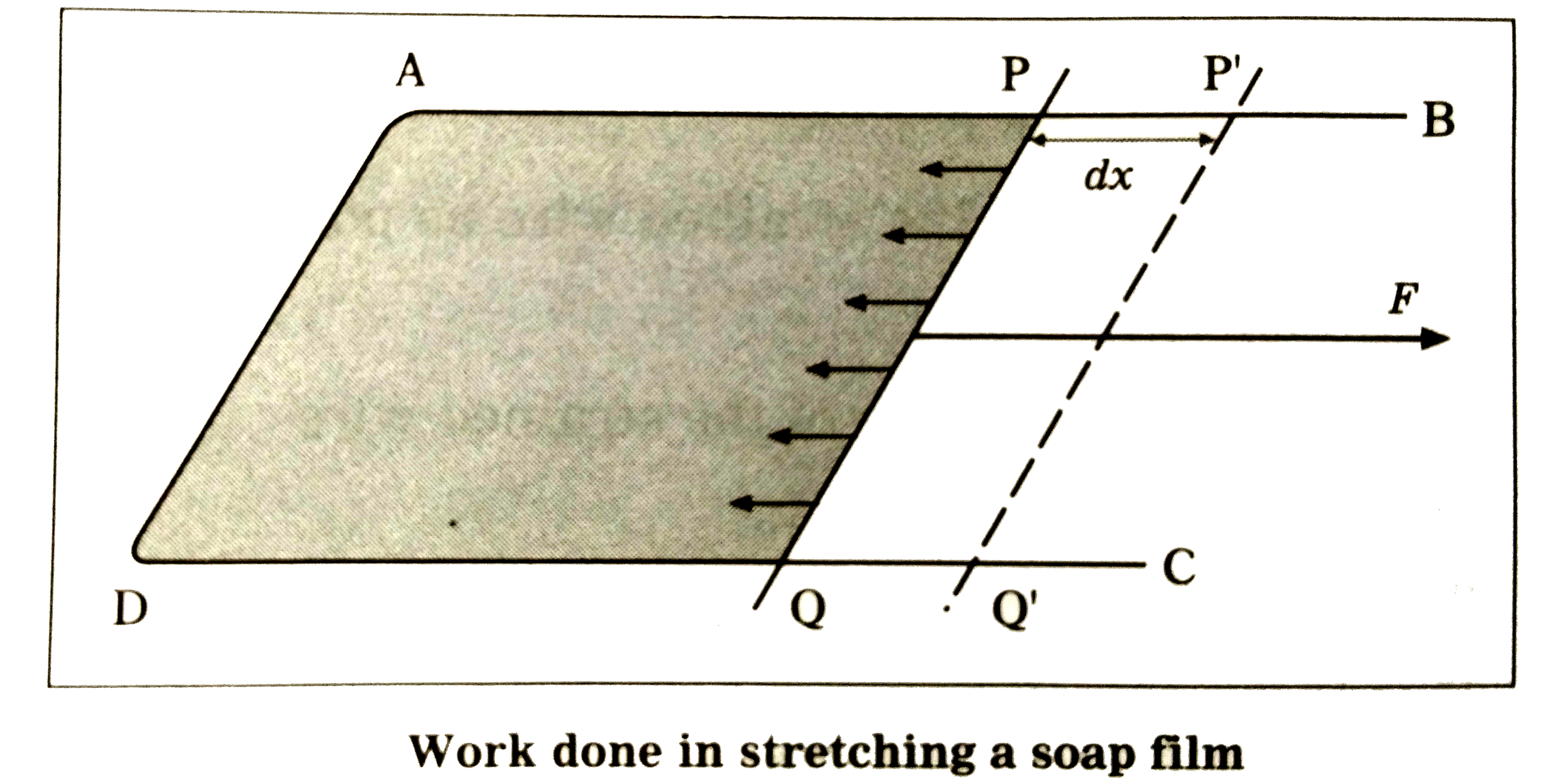
Text Solution
Verified by Experts
Topper's Solved these Questions
DERIVATIONS-I
NAVNEET PUBLICATION - MAHARASHTRA BOARD|Exercise Wave Motion|2 VideosDERIVATIONS-I
NAVNEET PUBLICATION - MAHARASHTRA BOARD|Exercise Stationary Waves|5 VideosDERIVATIONS-I
NAVNEET PUBLICATION - MAHARASHTRA BOARD|Exercise Elasticity|2 VideosDEFINITIONS
NAVNEET PUBLICATION - MAHARASHTRA BOARD|Exercise COMMUNICATION SYSTEMS|3 VideosDERIVATIONS-II
NAVNEET PUBLICATION - MAHARASHTRA BOARD|Exercise Assignments|9 Videos
Similar Questions
Explore conceptually related problems