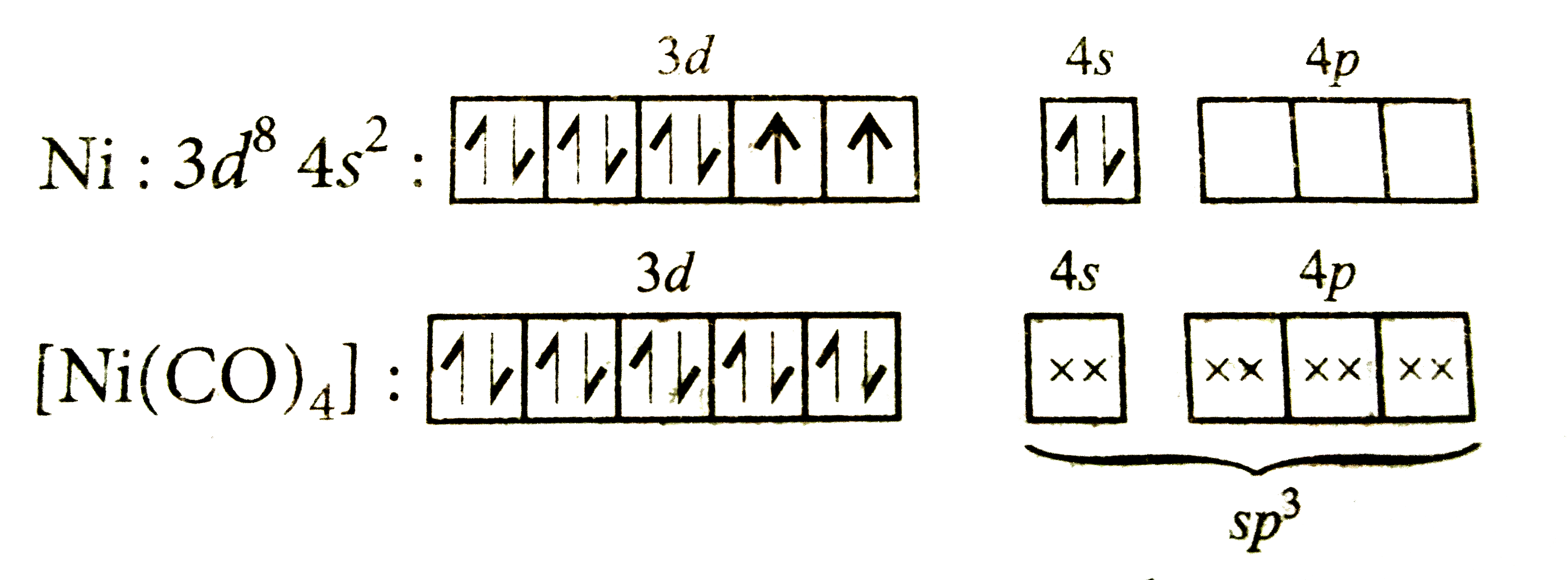
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
NCERT FINGERTIPS|Exercise Application Of Coordination Compounds|2 VideosCOORDINATION COMPOUNDS
NCERT FINGERTIPS|Exercise Higher Order Thinking Skills|9 VideosCOORDINATION COMPOUNDS
NCERT FINGERTIPS|Exercise Bonding In Coordination Compounds|40 VideosCHEMISTRY IN EVERYDAY LIFE
NCERT FINGERTIPS|Exercise Assertion And Reason|15 VideosELECTROCHEMISTRY
NCERT FINGERTIPS|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS-COORDINATION COMPOUNDS -Bonding In Metal Carbonyls
- The number of bridging CO ligand(s) and Co-Co bond(s) in Co(2)(CO)(8...
Text Solution
|
- Ni(CO)(4) is
Text Solution
|
- The oxidation state of chromium in Cr(CO)(6) is
Text Solution
|
- The correct structure of Fe (CO)(5) is ?
Text Solution
|
- Select the true statement from the following for metal carbonyls ?
Text Solution
|