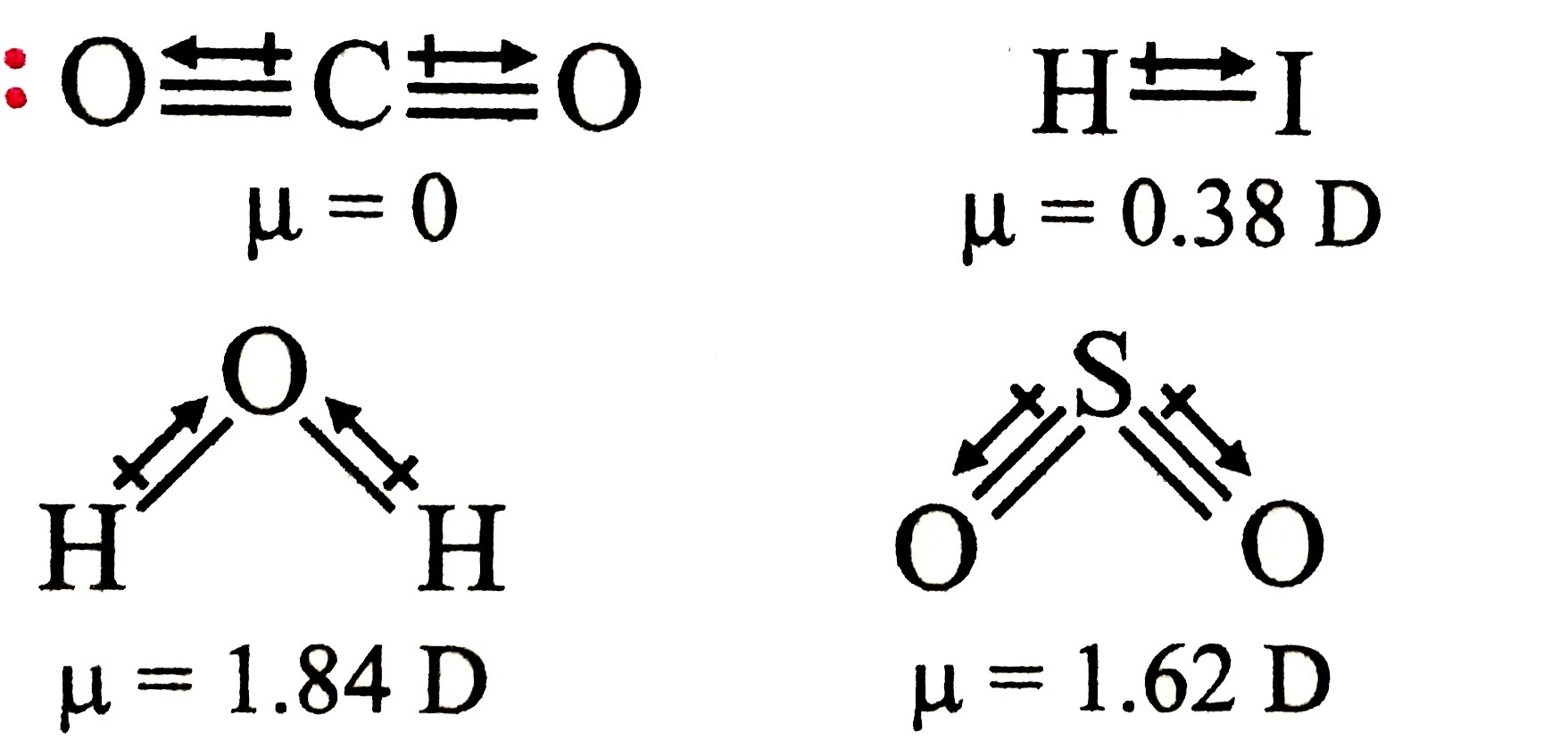
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS-CHEMICAL BONDING & MOLECULAR STRUCTURE-NCERT Exemplar
- Isostructrual species are those which have the same shape and hybridis...
Text Solution
|
- Polarity in a molecule and hence the dipole moment depends primarily o...
Text Solution
|
- The hybridisatipon of atomic orbitals of nitrogen in NO(2)^(+),NO(3)^(...
Text Solution
|
- Hydrogen bonds are formed in many compounds e.g. H(2)O, HF, NH(3). The...
Text Solution
|
- In PO(4)^(3-) ion the formal charge on the oxygen atom of P-O bond is
Text Solution
|
- In NO(3)^(-) ion, the number of bond pairs and lone pairs of electrons...
Text Solution
|
- Which of the following species has tetrahedral geometry?
Text Solution
|
- Number of pi bonds and sigma bonds in the following structure is
Text Solution
|
- Which molecule/ion out of the following does not contain unpaired elec...
Text Solution
|
- In which of the following molecule/ion all the bonds are not equal?
Text Solution
|
- In which of the following substances will hydrogen bond be strongest?
Text Solution
|
- If the electron configuration of an element is 1s^(2), 2s^(2), 2p^(6),...
Text Solution
|
- Which of the following angle corresponds to sp^(2) hydridisation ?
Text Solution
|
- Which of the following orderof energies of molecular orbitals of N(2) ...
Text Solution
|
- Which of the following statement is not correct from the view point of...
Text Solution
|