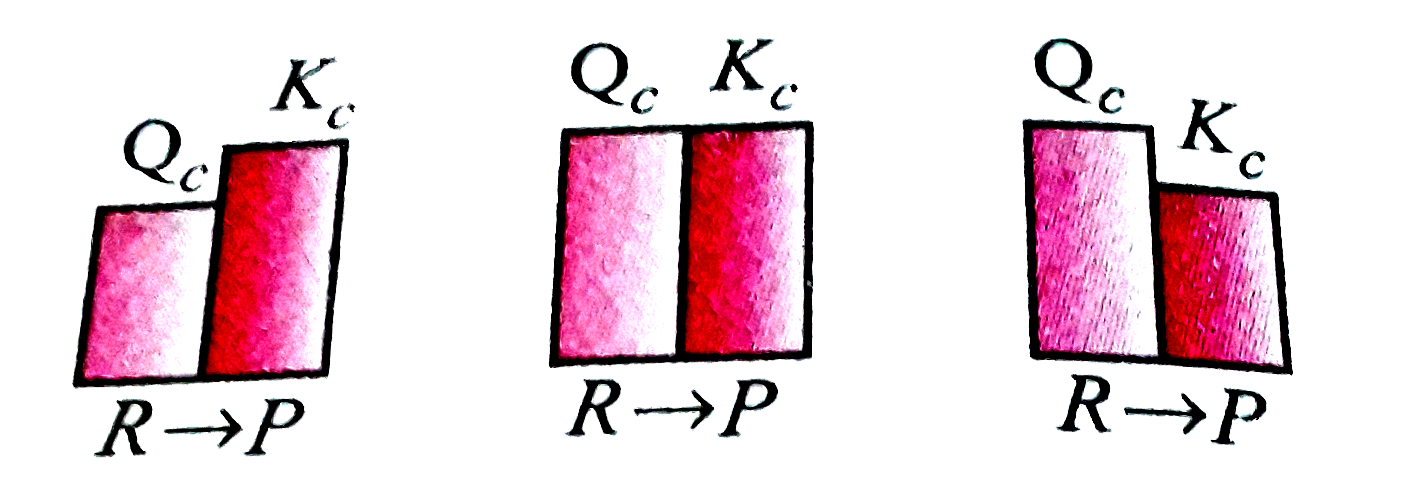A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
EQUILIBRIUM
NCERT FINGERTIPS|Exercise Relation Between Equilibrium Constant Constant, Reaction Quotient And Gibbs Energy|2 VideosEQUILIBRIUM
NCERT FINGERTIPS|Exercise Factors Affecting Equilibria|10 VideosEQUILIBRIUM
NCERT FINGERTIPS|Exercise Heterogeneous Equilibrium|6 VideosENVIRONMENTAL CHEMISTRY
NCERT FINGERTIPS|Exercise Assertion And Reason|15 VideosHYDROCARBONS
NCERT FINGERTIPS|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS-EQUILIBRIUM -Applications Of Equilibrium Constant
- N(2)O(4(g))rArr2NO(2),K(c)5.7xx10^(-9) at 298 K At equilibrium :-
Text Solution
|
- Study the figure below and mark the correct statement about K(c) and d...
Text Solution
|
- Predict the direction of the reaction from comparison of Q(c) and K(c)...
Text Solution
|
- In the following reaction: 2NO((g))+Cl(2(g))hArr2NOCl((g)) it is o...
Text Solution
|
- 0.6 moles of PCl(5), 0.3 mole of PCl(3) and 0.5 mole of Cl(2) are ...
Text Solution
|