A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLUTIONS AND COLLIGATIVE PROPERTIES
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS|Exercise Exercise 2 (Miscellaneous Problems)|57 VideosSOLUTIONS AND COLLIGATIVE PROPERTIES
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS|Exercise MHT CET Corner|25 VideosSOLUTIONS AND COLLIGATIVE PROPERTIES
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS|Exercise MHT CET Corner|25 VideosSOLID STATE
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS|Exercise MHT CET CORNER|12 VideosSOME BASIC CONCEPTS OF CHEMISTRY
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS|Exercise EXERCISE 2|30 Videos
Similar Questions
Explore conceptually related problems
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS-SOLUTIONS AND COLLIGATIVE PROPERTIES -Exercise 1 (Types of Solutions and Concentration of Solutions)
- Vapour pressure of C CL(4) at 25^@C is 143 mmHg 0.05g of a non-volatil...
Text Solution
|
- The vapour pressure of two liquids P and Q are 100 and 50 torr, respec...
Text Solution
|
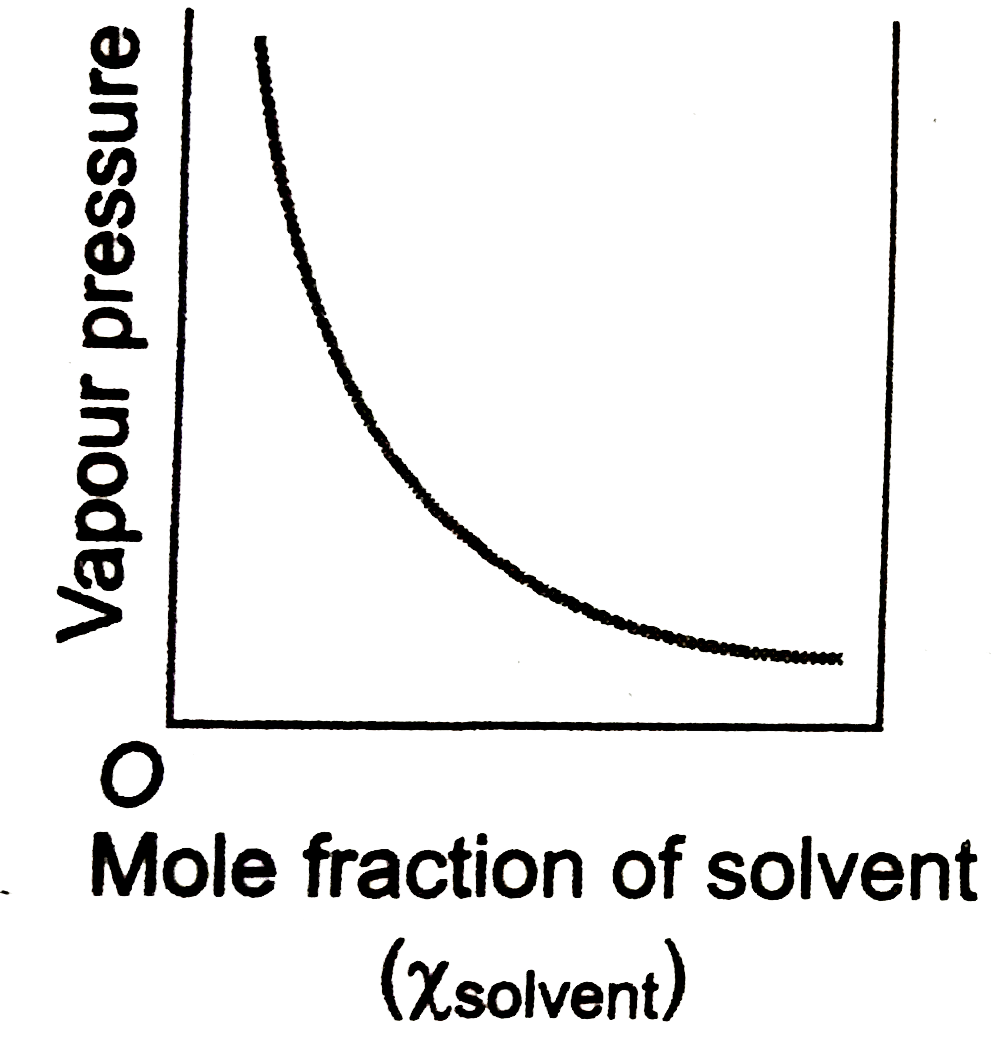
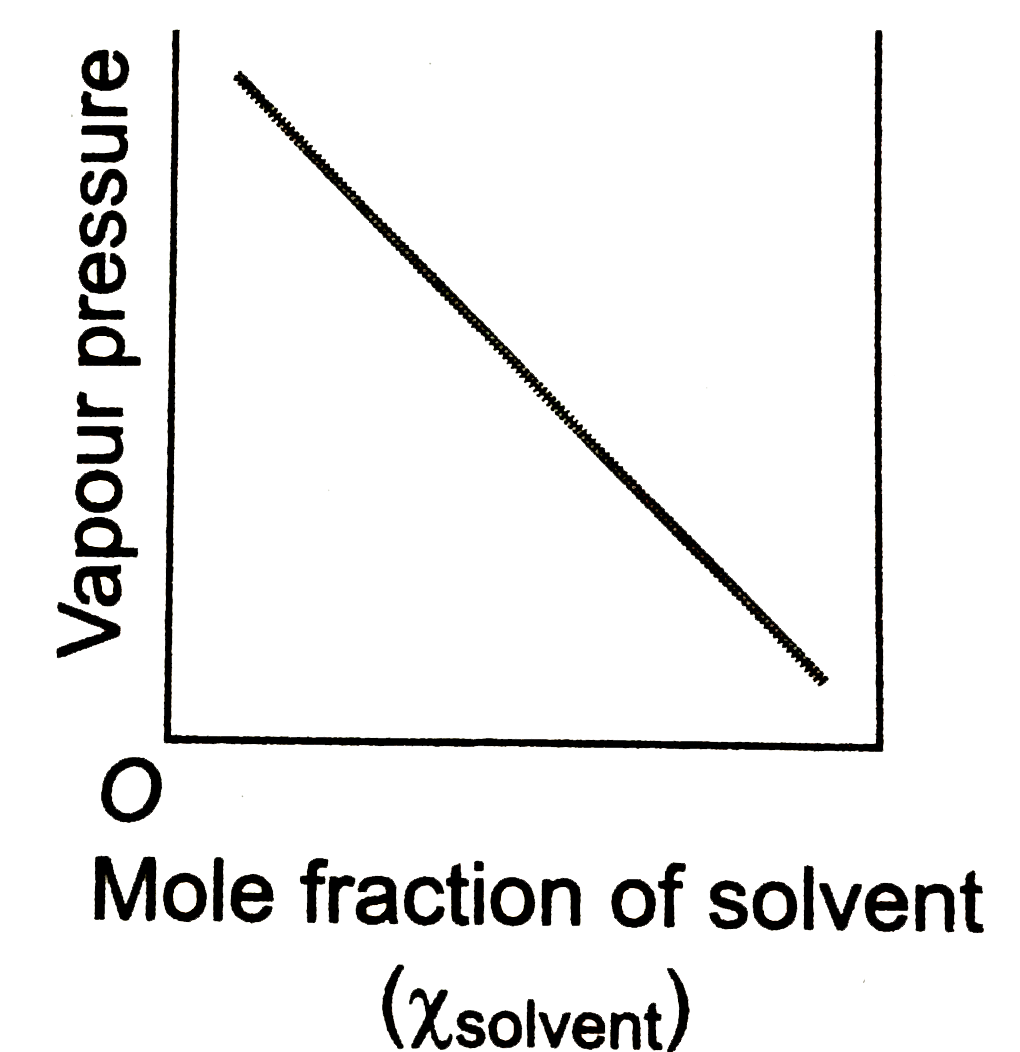
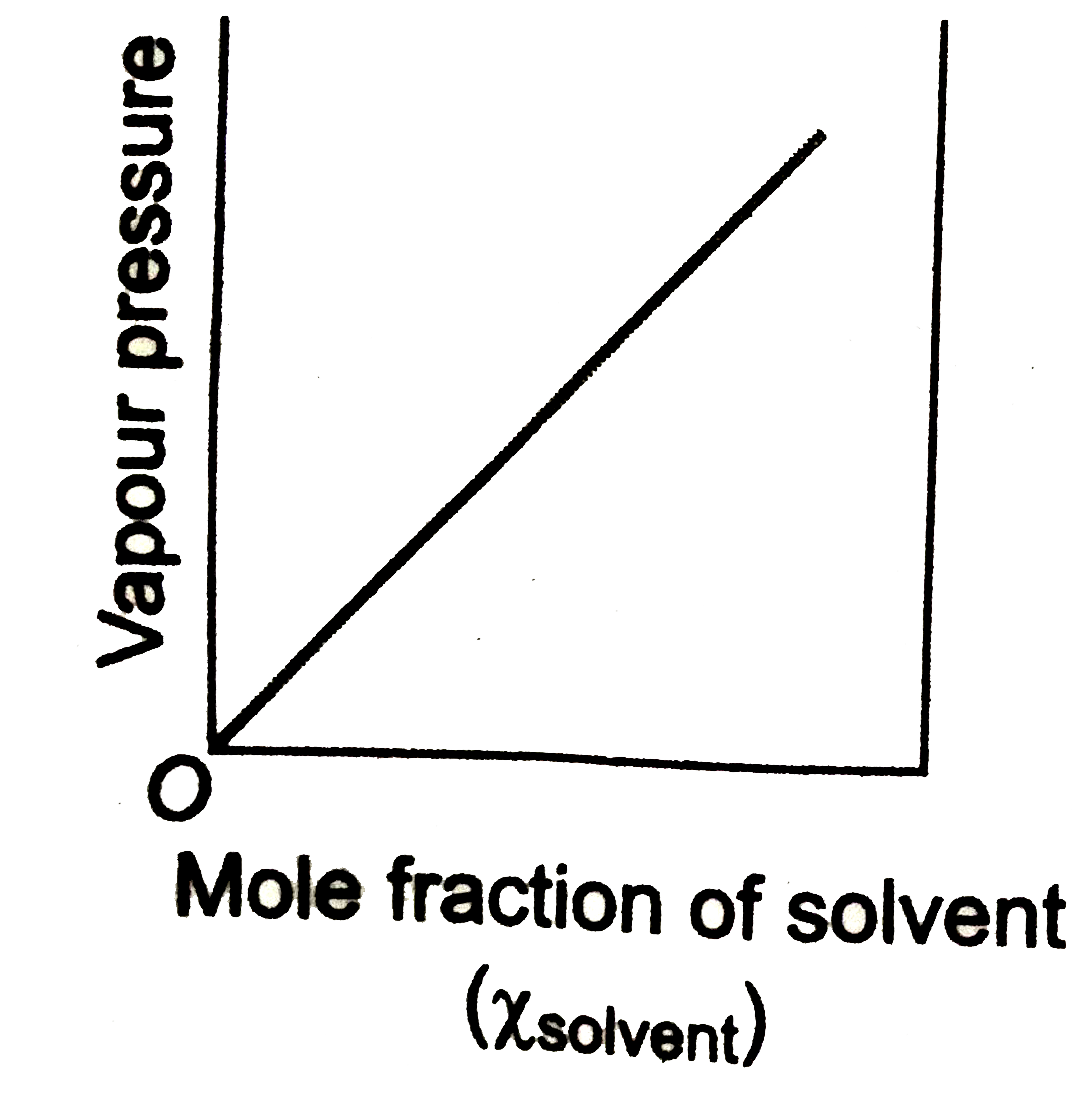
- Which of the following plot obeys the Raoult's law for all concentrati...
Text Solution
|
- At 40^(@)C the vapour pressure of pure liquids, benzene and toluene, a...
Text Solution
|
- The solution which shows large positive deviation from Raoult's law fo...
Text Solution
|
- 1xx10^(3)m" solution of Pt "(NH(3))(4)Cl(4) " in "H(2)O shows depress...
Text Solution
|
- What happens to freezing point of benzene when nephthalens is added ?
Text Solution
|
- A 5.25% solution of a substance is isotonic with a 1.5% solution of ur...
Text Solution
|
- The difference between the boiling point and freezing point of an aque...
Text Solution
|
- A 6% of urea is isotonic with
Text Solution
|
- Which of the following is not a colligative property?
Text Solution
|
- In an osmotic pressure measurement experiment, a 5% solution of compou...
Text Solution
|
- A solution of urea boils at 100.18^(@)C at the atmospheric pressure. ...
Text Solution
|
- The molal elevation constant of water =0.52 K m^(-1). The boiling poin...
Text Solution
|
- A solution containing 4g of polyvinyl chloride polymer in 1 L of dioxa...
Text Solution
|
- If alpha is the degree of dissociation of Na(2)SO(4) the van't Hoff's ...
Text Solution
|
- At 25^(@)C, the highest osmotic pressure is exhibited by 0.1 M solutio...
Text Solution
|
- Calculate the molal depression constant of a solvent which has freezin...
Text Solution
|
- The freezing point of 1% of lead nitrate solution in water will be :
Text Solution
|
- The relative lowering of vapour pressure of an aqueous solution contai...
Text Solution
|