A
B
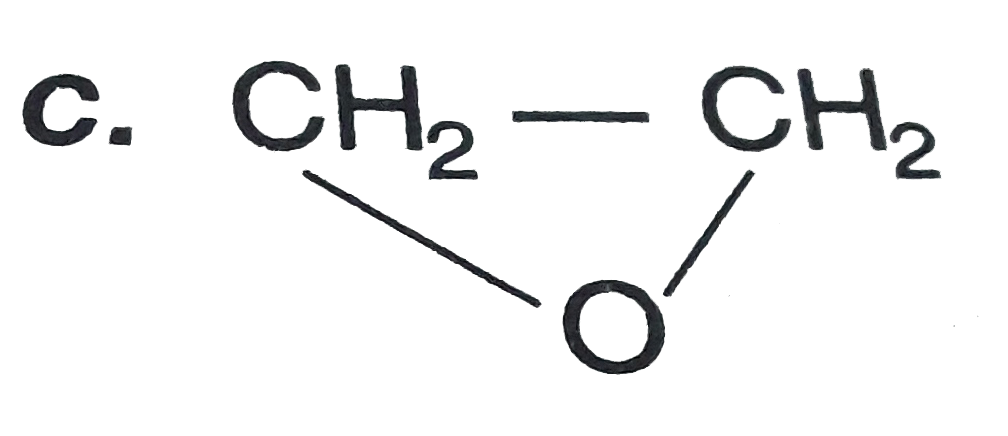
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALCOHOLS, PHENOLS AND ETHERS
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS|Exercise MHT CET Corner|33 VideosALCOHOLS, PHENOLS AND ETHERS
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS|Exercise PRACTICE EXERCISE (Exercise 1 (TOPICAL PROBLEMS))|119 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS|Exercise MHT CET CORNER|21 Videos
Similar Questions
Explore conceptually related problems
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS-ALCOHOLS, PHENOLS AND ETHERS-PRACTICE EXERCISE (Exercise 2 (MISCELLANEOUS PROBLEMS))
- A and B respectively are
Text Solution
|
- The main product of the following reaction is C(6)H(5)CH(2)CH(OH)CH(...
Text Solution
|
- C(2)H(4)O(A) reacts with CH(3)MgBr followed by decomposition with H(3)...
Text Solution
|
- Boiling point of CH(3)CH(2)OH(351 K) is much higher than that of isome...
Text Solution
|
- In the following sequence of reactions, CH(3)CH(2)OHoverset(P+I(2))(...
Text Solution
|
- Which of the following statement is correct for the following dehydrat...
Text Solution
|
- An organic compound 'X' on treatment with pyridinium chlorochromate in...
Text Solution
|
- CH(3)-overset(CH(3))overset("| ")underset(CH(3))underset("| ")"C "CH...
Text Solution
|
- The dehydration of 2-Methyl butanol with conc. H(2)SO(4) gives
Text Solution
|
Text Solution
|
- Phenol is heated with a solution of mixture of KBr and KBrO(3). The ma...
Text Solution
|
- A is
Text Solution
|
- Chlorobenzene overset("Reaction")underset(X)rarr "Phenol" overset("Rea...
Text Solution
|
- (C(2)H(5)O)(2)CO underset(H(3)O^(+))overset(CH(3)MgBr("excess"))rarrA....
Text Solution
|
- The following reaction is known as
Text Solution
|
Text Solution
|
- Dehydration of alcohols, will be in the order
Text Solution
|
- The structure of the compound that gives a tribromo derivative on trea...
Text Solution
|
- The product of the above reaction is
Text Solution
|
- Arrange the following compounds in increasing order of boiling point :...
Text Solution
|