Text Solution
Verified by Experts
Topper's Solved these Questions
SOLUTIONS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise LONG ANSWER QUESTIONS|10 VideosSOLUTIONS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEXT QUESTIONS|10 VideosSOLUTIONS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise VERY SHORT ANSWER QUESTIONS|29 VideosSOLID STATE
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEXT QUESTIONS|27 VideosSURFACE CHEMISTRY
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEXT QUESTIONS|8 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-SOLUTIONS -SHORT ANWER QUESTIONS
- A solution of glucose in water is labelled as 10% w/w. What would be t...
Text Solution
|
- A solution of sucrose in water is labelled as 20% w/w. What would be t...
Text Solution
|
- How many ml of 0.1 HCl is required to react completely with 1.0g mixtu...
Text Solution
|
- A solution is obtained by mixing 300g of 25% solution and 400g of 40% ...
Text Solution
|
- An antifreeze solution is prepared from 222.6g of ethylene glycol [(C(...
Text Solution
|
- Why do gases always tend to be less soluble in liquids as the temperat...
Text Solution
|
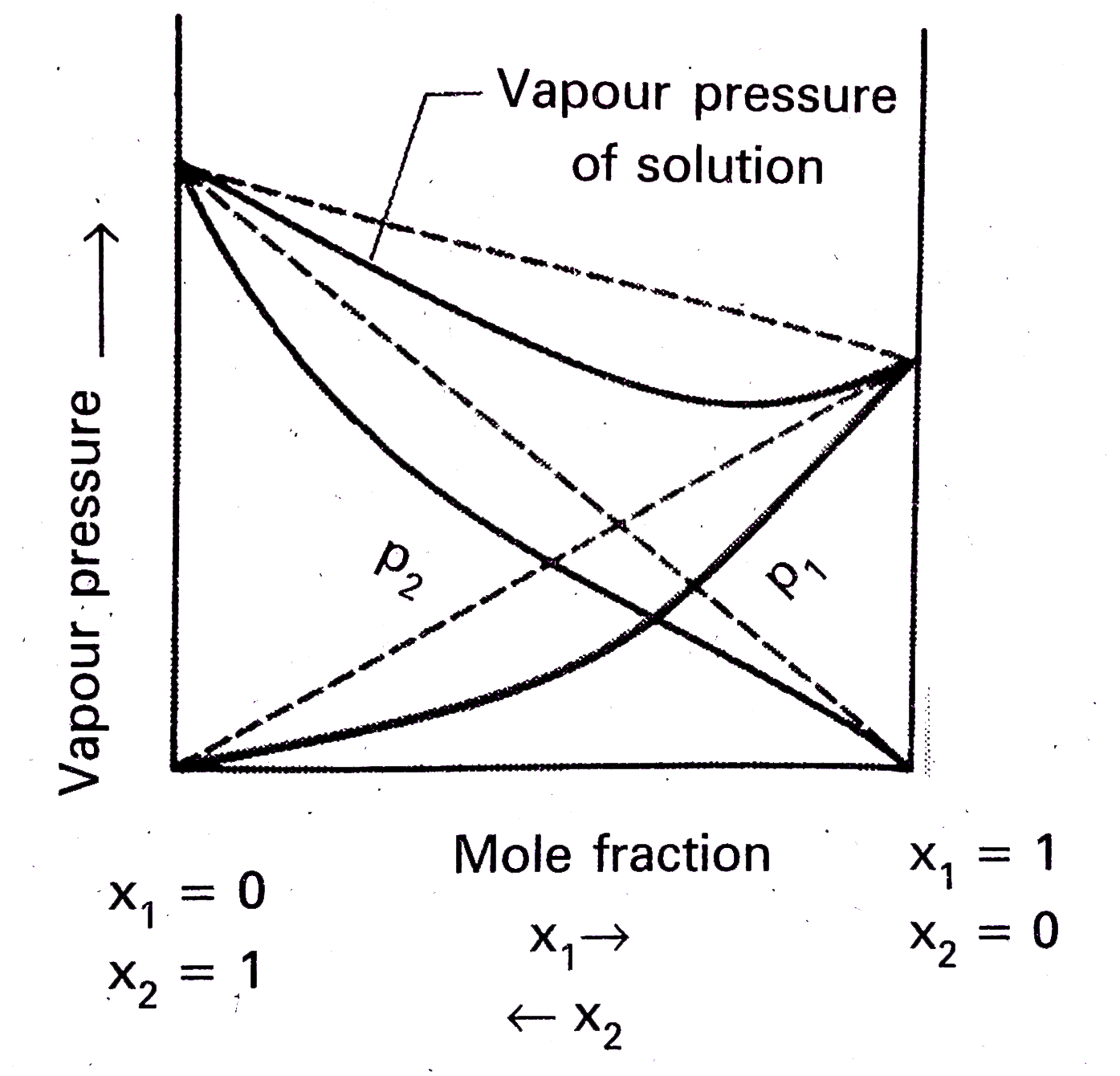
- What is meant by positive deviations from Raoult's law and how is the ...
Text Solution
|
- What is meant by negative deviation from Raoult's law and how is the s...
Text Solution
|
- The vapour pressure of water is 12.3 k P(a) at 300 K. Calculate the va...
Text Solution
|
- Calculate the mass of a non-volatile solute (molar mass 40"g mol"^(-1)...
Text Solution
|
- A 5% solution (by mass) of cane suger in water has freezing point of 2...
Text Solution
|
- If the osmotic pressure of glucose solution is 1.52 bar at 300 K. What...
Text Solution
|
- Vapour pressure of of water at 293K is 17.535 mm Hg. Calculate the vap...
Text Solution
|
- How is molar mass related to the elevation in boiling point of a solut...
Text Solution
|
- What is an ideal solution ?
Text Solution
|
- What is relative lowering of vapour pressure ? How is it useful to det...
Text Solution
|
- How is molar mass related to the depression in freezing point of a sol...
Text Solution
|
- The vapour pressure of a solution containing non volatile solute is le...
Text Solution
|
- Vapour pressure of pure water at 298 K is 23.8 mm Hg. 50g urea (NH(2)C...
Text Solution
|
- Calculate the vapour pressure of a solution containing 9g of glucose i...
Text Solution
|