Text Solution
Verified by Experts
Topper's Solved these Questions
ELECTROCHEMISTRY & CHEMICAL KINETICS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise VERY SHORT ANSWER QUESTIONS|63 VideosELECTROCHEMISTRY & CHEMICAL KINETICS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise SHORT ANSWER QUESTIONS|25 VideosELECTROCHEMISTRY & CHEMICAL KINETICS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise DAM SURE|18 VideosD AND F- BLOCK ELEMENTS AND CO-ORDINATION COMPOUNDS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEXT QUESTIONS|33 VideosGENERAL PRINCIPLES OF MMETALLURGY
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise DAM SURE SAQ|1 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-ELECTROCHEMISTRY & CHEMICAL KINETICS-PROBLEMS
- From the rate expression for the following reactions, determine their ...
Text Solution
|
- For the reaction 2A+B to A, B, the rate =K[A] [B]^(2) with k= 2.0x 10^...
Text Solution
|
- The decomposition of NH(3) on platjinum surface is zero order reaction...
Text Solution
|
- The rate expression for the decomposition of dimethyl ether in terms o...
Text Solution
|
- A reaction is second order with respect to a reactant. How is the rate...
Text Solution
|
- A reaction is first order in A and second order in B. i) Write the d...
Text Solution
|
- In a reactio between A and B, the initial rate of reaction (r(0)) was ...
Text Solution
|
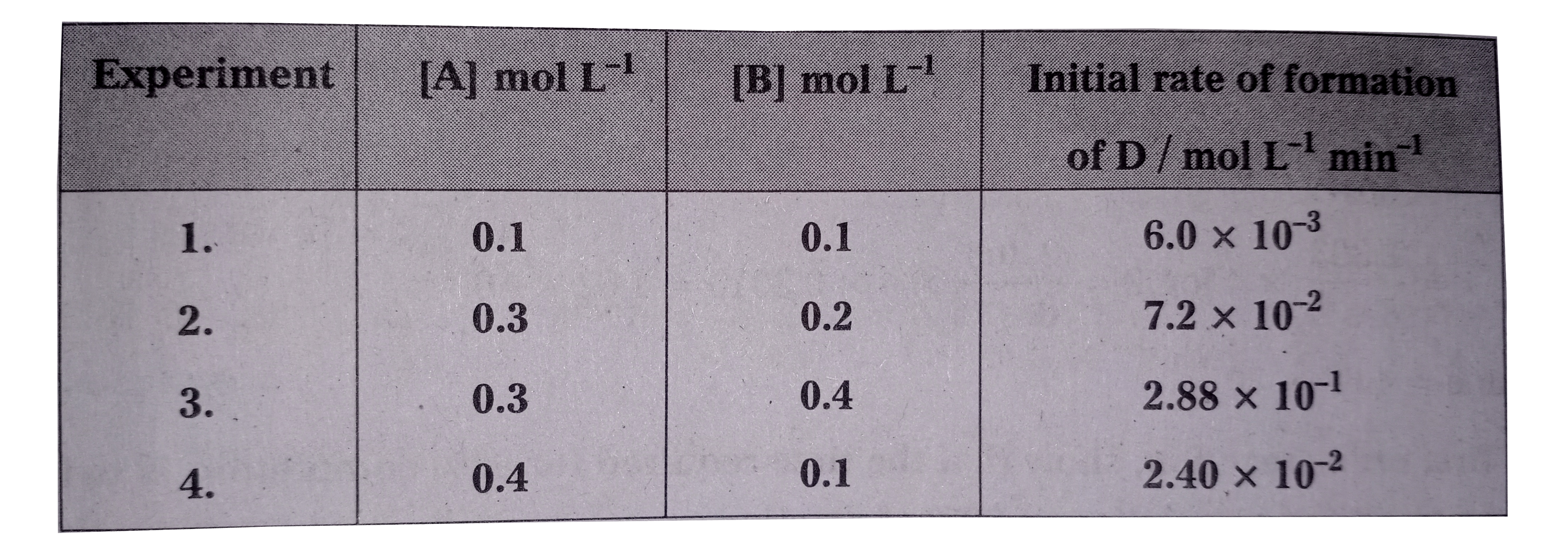
- The following results have been obtained during the kinetic studies of...
Text Solution
|
- The rate constant for a first order reaction is 60 s^(-1). How much ti...
Text Solution
|
- For a first order reaction,k show that the time required for 99% compl...
Text Solution
|
- For the decomposition of azosiopropane to hexane and nitrogen at 543 k...
Text Solution
|
- The following data were obtained during the first order thermal decomp...
Text Solution
|
- The rate constant for the decomposition of hydrocarbons is 2.418 xx10^...
Text Solution
|
- Consider a certain reaction A to Products with k=2.0 xx10^(-2)s^(-1). ...
Text Solution
|
- Sucrose decompose in acid solution into glucose and fructose accordin...
Text Solution
|
- The decomposition of hydrocarbon follows the equation K=(4.5xx10^(11...
Text Solution
|
- The rate constant for the first order decomposition of H(2)O(2) is giv...
Text Solution
|
- The decomposition of A into product has value of k as 4.5 xx10^(3) s^(...
Text Solution
|
- The time required for 10% completion of a first order reaction at 298 ...
Text Solution
|
- The rate of a reaction quadruples when temperature charges from 293 K ...
Text Solution
|