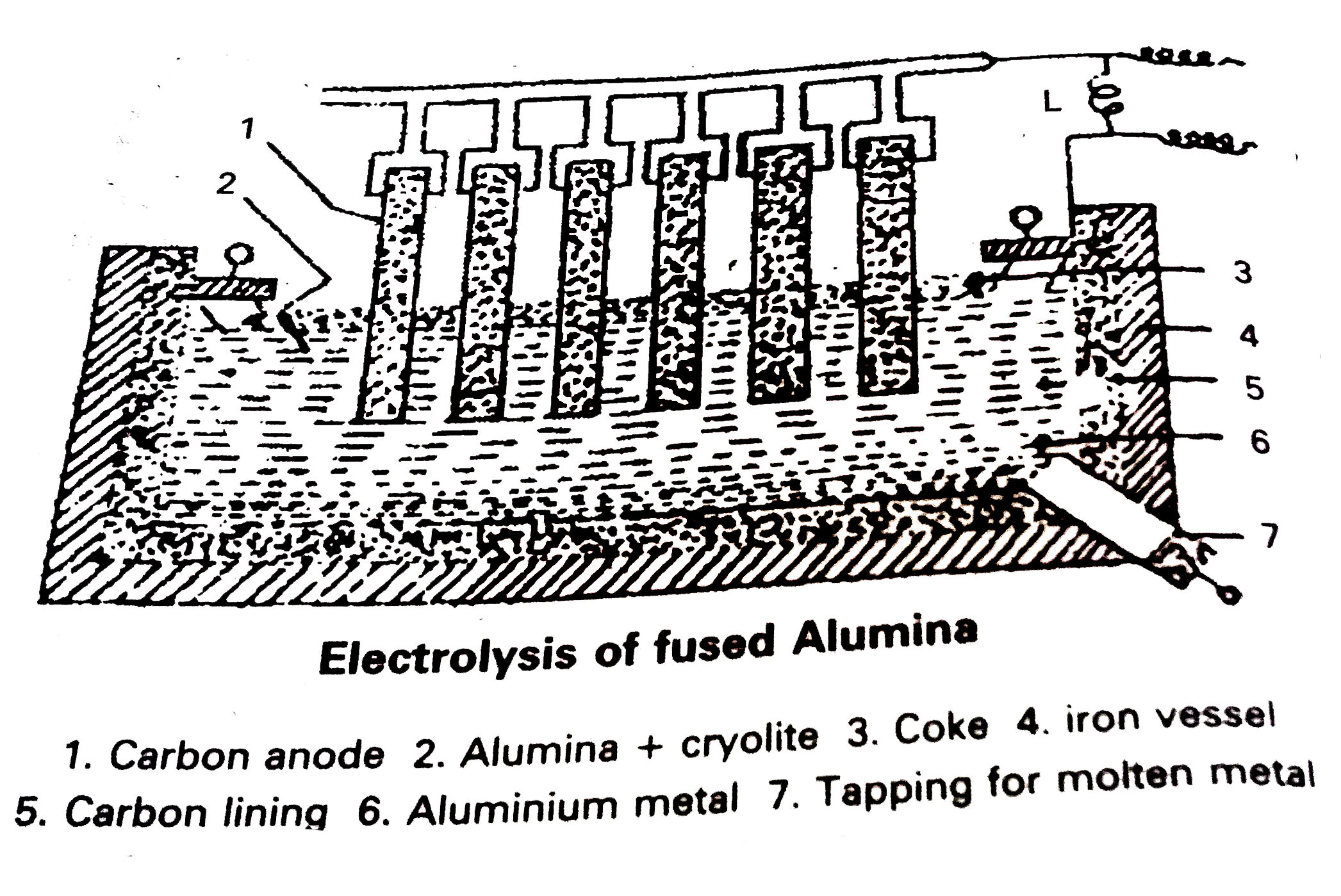Extraction of Aluminium from Bauxite :
For the purpose of extraction of Al, Bauxite is the best source.
Purification of Bauxite : Bauxite containing `Fe_(2)O_(3)` as impurity is known as red Bauxite . Bauxite containing `SiO_(2)` as impurity is known as white Bauxite and can be purified by "Serpeck's Process" . Red Bauxite is purified by Bayer's process and Hall's process.
Bayer's Process : Red bauxite is roasted and digested in concentrated NaOH at 423 K. Bauxite dissolves in NaoH to from sodium meta aluminate while impurity `Fe_(2)O_(3)` does not dissolve which can be removed by filtration.
`Al_(2)O_(3).2H_(2)O+2NaOHrarr2NaAlO_(2)+3H_(2)O`
The solution which contains sodium meta aluminate is diluted and crystals of `Al(OH)_(3)` , are added which serves as seeding orgent. Sodium meta aluminate undergoes to precipitate `Al(OH)_(3)`
`2NaAlO_(2)+4H_(2)Orarr2NaOH+2Al(OH)_(3)darr`
`Al(OH)_(3)` is filtered and ignited at `1200^(@)C` to get anhydrous alumina.
`2Al(OH)_(3)overset(Delta)rarrAl_(2)O_(3)+3H_(2)O`
Halls' Process : Red Bauxite is fused with sodium carbonate to from sodium meta aluminate which is extracted with water. The impurity `Fe_(2)O_(3)` is filtered out.
`Al_(2)O_(3)+Na_(2)CO_(3)rarr2NaAlO_(2)+CO_(2)`
Into the solutionof sudium meta aluminate, `CO_(2)` gas is passed to precipitate `Al(OH)_(3)`
`2Al(OH)_(3)+3H_(2)O+CO_(2)overset(475K)rarr2Al(OH)_(3)darrNa_(2)CO_(3)`
The precipitated `Al(OH)_(3)` is ignited at `1200^(@)C` to get anhydrous alumina .
`2Al(OH)_(3)rarrAl_(2)O_(3)+3H_(2)O`
Serpeck's process :Powdered bauxite is mixed with coke and heated to 2075 K in a current of introgen gas . Aluminium Nitride is formed while `SiO_(2)` is reduced to si which escapes out.
`Al_(2)O_(3)+3C+N_(2)overset(2075K)rarr2AlN+3COuarr`
`SiO_(2)+2CrarrSirarr+2COuarr`
Aluminium nirtride is hydrolysed to get aluminium hydroxide which on ignition gives anhydrous alumina.
`AlN+3H_(2)OrarrAl(OH)_(3)darr+NH_(3)uarr`
`2Al(OH)_(3)overset(Delta)rarrAl_(2)O_(3)+3H_(2)O`
Electrolytic Reduction of alumina : Pure Alumina `(Al_(2)O_(3))` is a bad conductor of electricity and it has high melting point `(2050^(@)C)` . So it cannot be electrolysed . Alumina is electrolysed by dissolving in fused cryolite to increase the conuctivity and small amount of Fluorspar is added to reduce its melt-ing point .Thus the electrolyte is a fused mixture of Alumina, Cryolite and Fluorspar.
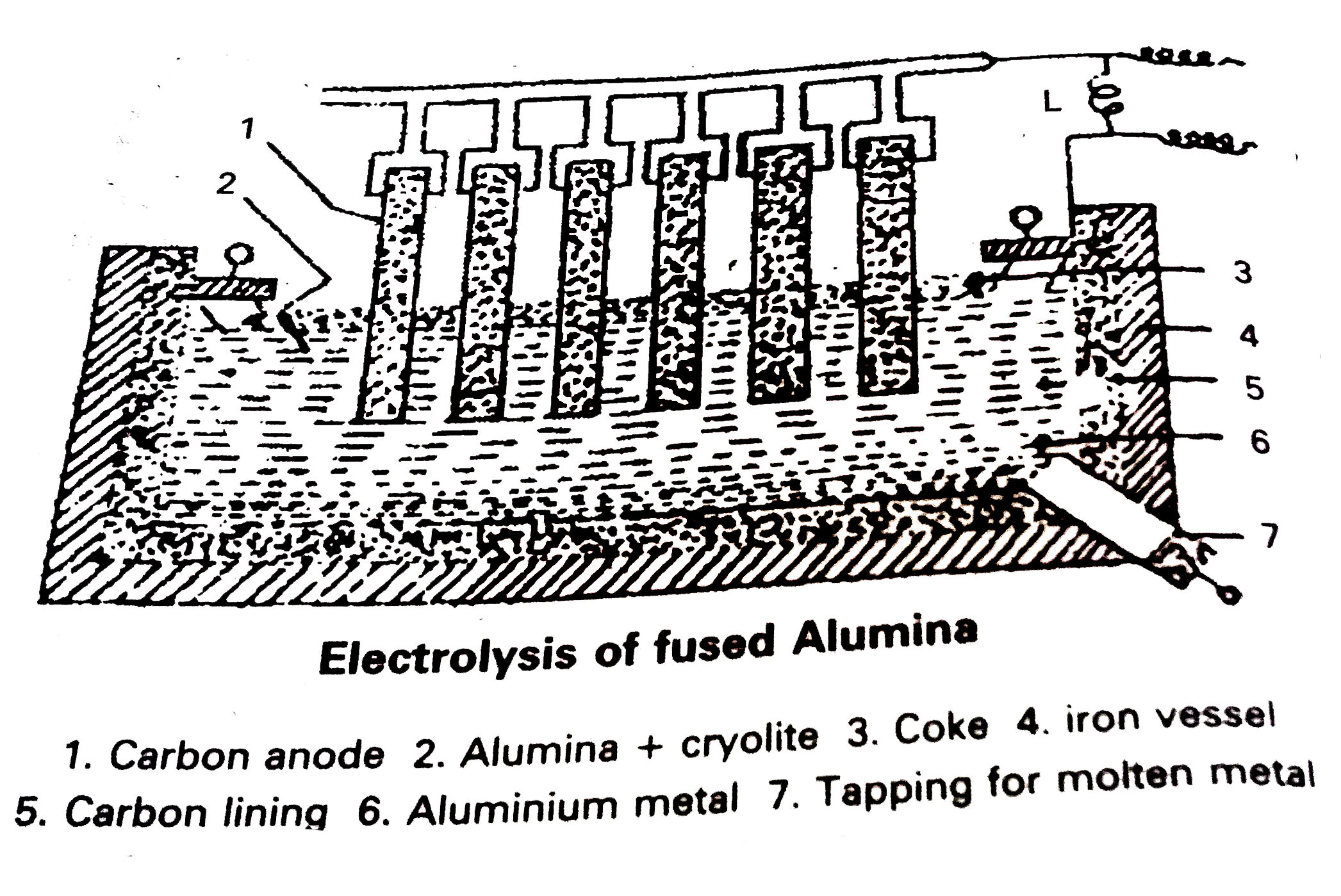
Electrolysis is carried out in an iron tank lined inside with graphite (carbon)which functions as cathode. A number of carbon rods (or) copper roda suspened in the electrolyte functions as Anode.
An electric current of 100 amperes at 6 to 7 volts is passsed through the electrolyte . Heat produced by the current keeps the mass in fused state at 1175 to 1225K . The following reactions take place in the electrolytic cell under these conditions .
`{:(Na_(3)AlF_(6)rarr3NaF+AlF_(3)),("Cryolite"),(4AlF_(3)rarr4Al^(3)+12F^(-)),("At cathode :"4Al^(3)+12e^(-)rarr4Al),("At anode : "12F^(-)rarr6F_(2)+12e^(-)):}`
`F_(2)` formed at the anode reacts with alumina and forms Aluminium fluoride.
`2Al_(2)O_(3)+6F_(2)rarr4AlF_(3)+3O_(2)`
Aluminium , produced at thr cathode, sinks to the bottom of the cell. It is removed from time to time through topping hole.
Purification of Aluminium : (Hoope's Process) The impurities are Si,Cu, Mn etc.,
The electrolytic cell used for refining of aluminium consists of iron tank lined inside with carbon .This acts as anode. The tank contains three of fused masses . The bottom layer contains impure aluminium. Middle layer contains mixture of `AlF_(3)` NaF and `BaF_(2) ` saturated with `Al_(2)O_(3)` Top layer contains pure aluminium and graphite rods kept in it act as cathode.

On passing current aluminium ions from the middle layer are discharged at the cathode as pure aluminium . Equialent amount of aluminium from the bottom layer passes into middle layer.