Text Solution
Verified by Experts
Topper's Solved these Questions
P-BLOCK ELEMENTS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise GROUP - 16 ELEMENTS (VERY SHORT ANSWER QUESTIONS)|37 VideosP-BLOCK ELEMENTS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise GROUP - 17 ELEMENTS (VERY SHORT ANSWER QUESTIONS)|37 VideosP-BLOCK ELEMENTS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEXT QUESTIONS|30 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise DAM SURE (SAQ - 8 Marks)|15 VideosPOLYMERS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEXT QUESTIONS|6 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-P-BLOCK ELEMENTS -GROUP 15 ELEMENTS (VERY SHORT ANSWER QUESTIONS)
- What is allotropy ? Explain the different allotropic forms of phosphou...
Text Solution
|
- How do you account for the inert character of dinitrogen ?
Text Solution
|
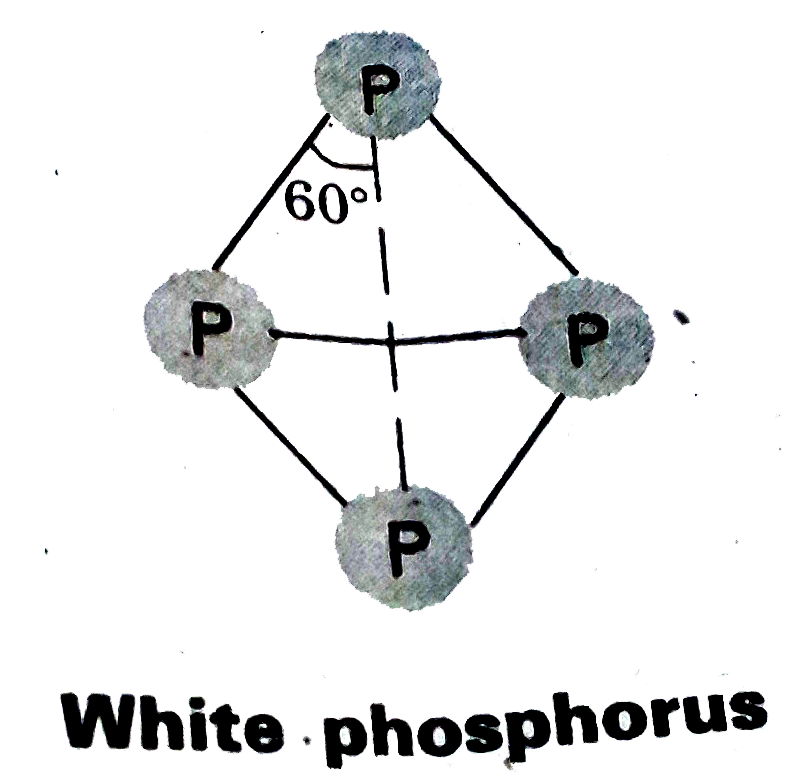
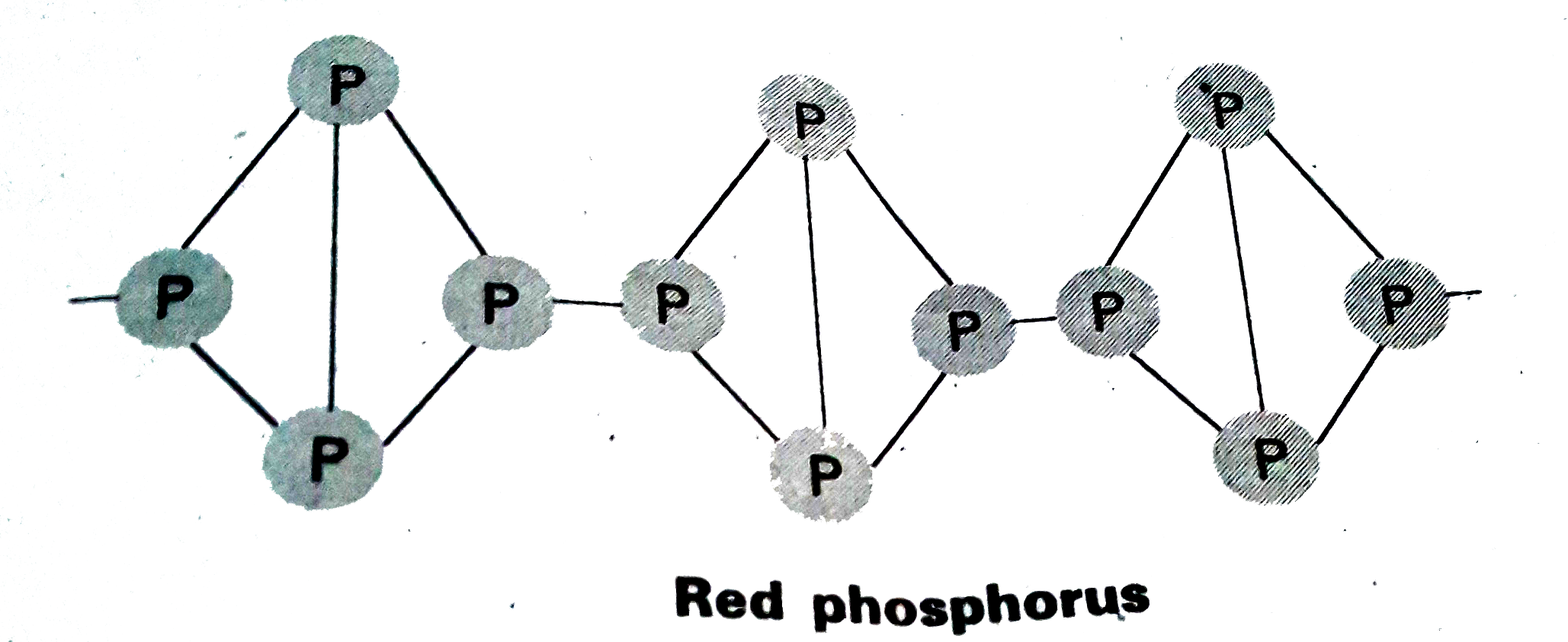
- Explain the difference in the structures of white and red phosphorus.
Text Solution
|
- How is alpha- black phosphorus prepared from red phosphorus ?
Text Solution
|
- Write the difference between the properties of white phosphorus and re...
Text Solution
|
- What is inert pair effect ?
Text Solution
|
- Explain why is NH(3) basic while "BiH"(3) is only feebly basic.
Text Solution
|
- Arrange the hydrides of group - 15 elements in the increasing order of...
Text Solution
|
- PH(3) is a weaker base than NH(3) - Explain.
Text Solution
|
- A hydride of group -15 elements dissolves in water to form a bbasic so...
Text Solution
|
- What happens when white phosphorus is heated with conc. NaOH solution ...
Text Solution
|
- NH(3) forms hydrogen bonds but PH(3) does not - why ?
Text Solution
|
- The HNH angle is higher than HPH, HAsH and HSbH angles - Why ?
Text Solution
|
- How do calcium phosphide and heavy water react ?
Text Solution
|
- Ammonia is a good complexing agent - Explain with an example.
Text Solution
|
- A mixture of "Ca"(3)"P"(2) and "CaC"(2) is used in making Holme's sign...
Text Solution
|
- Which chemical compound is formed in the brown ring test of nitrate io...
Text Solution
|
- Give the resonating structures of NO(2) and N(2)O(5).
Text Solution
|
- Why does R(3)P=O exist but R(3)B=O does not (R = alkyl group) ?
Text Solution
|
- How is nitric oxide (NO) prepared ?
Text Solution
|