Text Solution
Verified by Experts
Topper's Solved these Questions
P-BLOCK ELEMENTS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise GROUP - 16 ELEMENTS (VERY SHORT ANSWER QUESTIONS)|37 VideosP-BLOCK ELEMENTS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise GROUP - 17 ELEMENTS (VERY SHORT ANSWER QUESTIONS)|37 VideosP-BLOCK ELEMENTS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEXT QUESTIONS|30 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise DAM SURE (SAQ - 8 Marks)|15 VideosPOLYMERS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEXT QUESTIONS|6 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-P-BLOCK ELEMENTS -GROUP 15 ELEMENTS (VERY SHORT ANSWER QUESTIONS)
- Which oxide of nitrogen has oxidation number of N same as that in nitr...
Text Solution
|
- Write the chemical reactions that occur in the manufacture of nitric a...
Text Solution
|
- Iron becomes passive in conc. "HNO"(3) - Why ?
Text Solution
|
- Give the uses of a) nitric aicd and b) ammonia.
Text Solution
|
- What are the oxidation states of phosphorus in the following ? H(3)P...
Text Solution
|
- What are the oxidation states of phosphorus in the following ? "PCl"...
Text Solution
|
- What are the oxidation states of phosphorus in the following ? "Ca"(...
Text Solution
|
- What are the oxidation states of phosphorus in the following ? "Na"(...
Text Solution
|
- What are the oxidation states of phosphorus in the following ? "POF"...
Text Solution
|
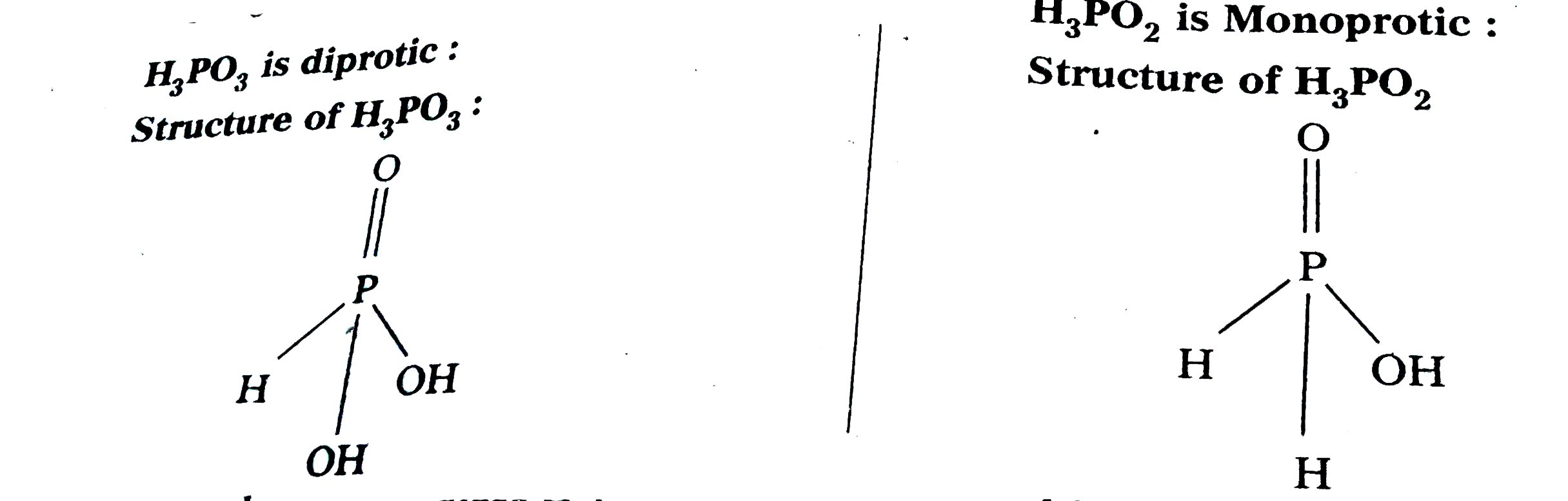
- H(3)"PO"(3) is diprotic while H(3)"PO"(2) is monoprotic - Why ?
Text Solution
|
- Give the disproportionation reaction of H(3)PO(3).
Text Solution
|
- H(3)PO(2) is a good reducing agent - Explain with an example.
Text Solution
|
- Draw the structures of Hypo phosphoric acid
Text Solution
|
- Draw the structures of Cyclic meta phosphoric acid.
Text Solution
|
- Give an example of neutral oxide of nitrogen.
Text Solution
|
- Give the paramagnetic oxides of nitrogen.
Text Solution
|
- Why is white phosphorus is more reactive than red phosphorus ?
Text Solution
|
- Why does PCl(3) fume in moisture ?
Text Solution
|
- Explain the following reaction of alkali with red phosphrous.
Text Solution
|
- Explain the following reaction between PCl(3)" and "H(2)O.
Text Solution
|