Text Solution
Verified by Experts
Topper's Solved these Questions
P-BLOCK ELEMENTS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise GROUP 15 ELEMENTS (SHORT ANSWER QUESTIONS)|21 VideosP-BLOCK ELEMENTS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise GROUP 16 ELEMENTS (SHORT ANSWER QUESTIONS)|21 VideosP-BLOCK ELEMENTS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise DAM SURE VSAQ - 2 Marks|8 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise DAM SURE (SAQ - 8 Marks)|15 VideosPOLYMERS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEXT QUESTIONS|6 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-P-BLOCK ELEMENTS -GROUP - 18 ELEMENTS (VERY SHORT ANSWER QUESTIONS)
- List out the uses of Neon.
Text Solution
|
- Write any two uses of argon.
Text Solution
|
- In modern diving apparatus, a mixture of He and O(2) is used - Why ?
Text Solution
|
- Helium is heavier than hydrogen. Yet helium is used (instead of H(2)) ...
Text Solution
|
- How is XeO(3) prepared ?
Text Solution
|
- Give the preparation of a) XeOF(4) and b) XeO(2)F(2)
Text Solution
|
- Explain the structure of XeO(3).
Text Solution
|
- Noble gases are inert - explain.
Text Solution
|
- Write the name and formula of the first noble gas compound prepared by...
Text Solution
|
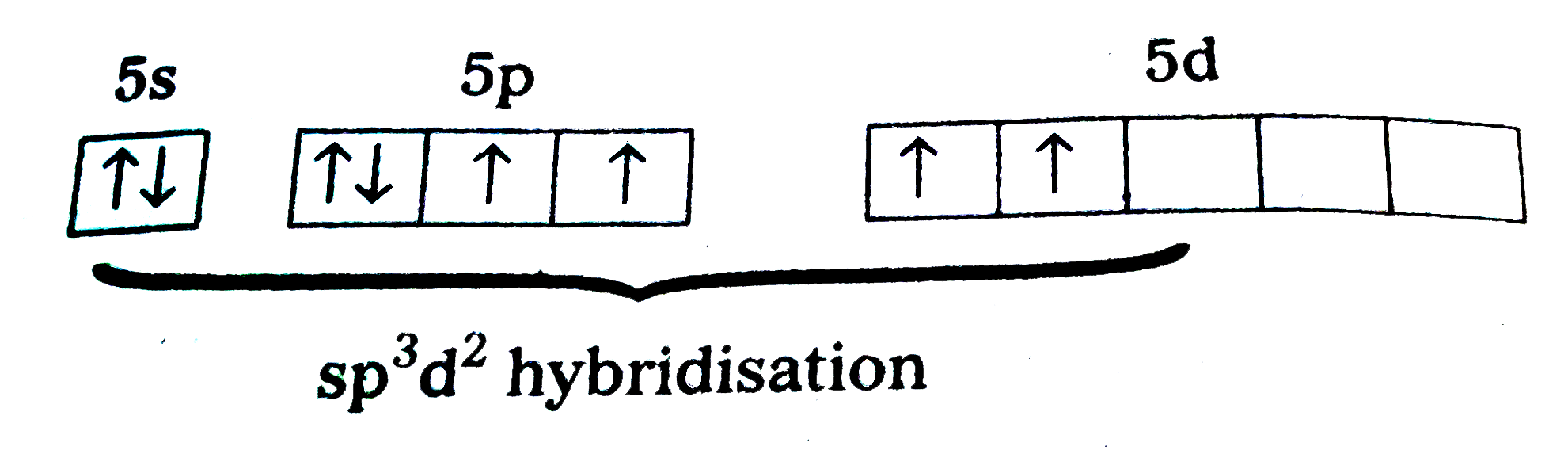
- Explain the shape of XeF(4) on the basis of VSEPR theory.
Text Solution
|
- Give the outer electronic configuration of noble gases.
Text Solution
|
- Why do noble gases form compounds with fluorine and oxygen only ?
Text Solution
|
- How is "XeOF"(4) prepared ? Describe its molecular shape.
Text Solution
|
- What is the major source of helium ?
Text Solution
|
- Which noble gas is radioactive ? How is it formed ?
Text Solution
|
- Name the following : most abundant noble gas in atmosphere
Text Solution
|
- Name the following : radioactive noble gas
Text Solution
|
- Name the following : noble gas with least boiling point
Text Solution
|
- Name the following : noble gas forming large number of compounds
Text Solution
|
- Name the following : noble gas not present in atmosphere
Text Solution
|