Text Solution
Verified by Experts
Topper's Solved these Questions
P-BLOCK ELEMENTS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise GROUP 17 ELEMENTS (SHORT ANSWER QUESTIONS)|10 VideosP-BLOCK ELEMENTS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise DAM SURE SAQ - 4 Marks|7 VideosP-BLOCK ELEMENTS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise GROUP 15 ELEMENTS (SHORT ANSWER QUESTIONS)|21 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise DAM SURE (SAQ - 8 Marks)|15 VideosPOLYMERS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEXT QUESTIONS|6 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-P-BLOCK ELEMENTS -GROUP 16 ELEMENTS (SHORT ANSWER QUESTIONS)
- Describe the manufacture of H(2)SO(4) by contact process.
Text Solution
|
- How is ozone prepared ? How does it react with the following ? PbS
Text Solution
|
- How is ozone prepared ? How does it react with the following ? KI
Text Solution
|
- How is ozone prepared ? How does it react with the following ? Hg
Text Solution
|
- How is ozone prepared ? How does it react with the following ? Ag
Text Solution
|
- Write a short note on the allotropy of sulphur.
Text Solution
|
- How does SO(2) react with the following ? Na(2)SO(3)("aq")
Text Solution
|
- How does SO(2) react with the following ? Cl(2)
Text Solution
|
- How does SO(2) react with the following ? "Fe"^(+3)"ions"
Text Solution
|
- How does SO(2) react with the following ? "KMnO"(4)
Text Solution
|
- Starting from elemental sulphur, how is H(2)SO(4) prepared ?
Text Solution
|
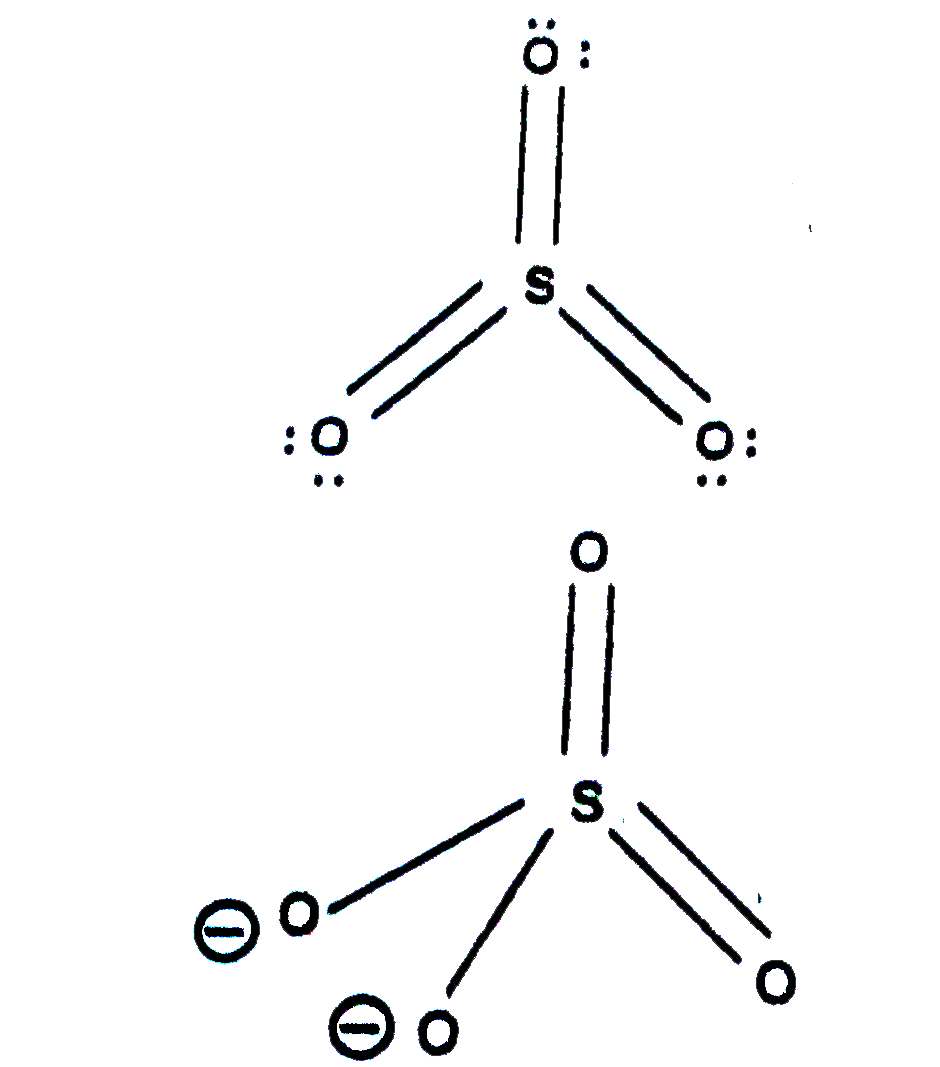
- Describe the structures (shapes) of SO(4)^(-2) and SO(3).
Text Solution
|
- Which oxide of sulphur can act as both oxidizing and reducing agent ?...
Text Solution
|
- Explain the conditions favourable for the formation of SO(3) from SO(2...
Text Solution
|
- Complete the following KCl+H(2)SO(4)to
Text Solution
|
- Complete the following "Sucrose"overset("Conc.H"(2)SO(4))to
Text Solution
|
- Complete the following Cu+H(2)SO(4)("Conc")to
Text Solution
|
- Complete the following C+H(2)SO(4)("Conc")to
Text Solution
|
- Which is used for drying ammonia ?
Text Solution
|
- Why conc "H"(2)"SO"(4),"P"(4)"O"(10) and anhydrous CaCl(2) cannot be u...
Text Solution
|