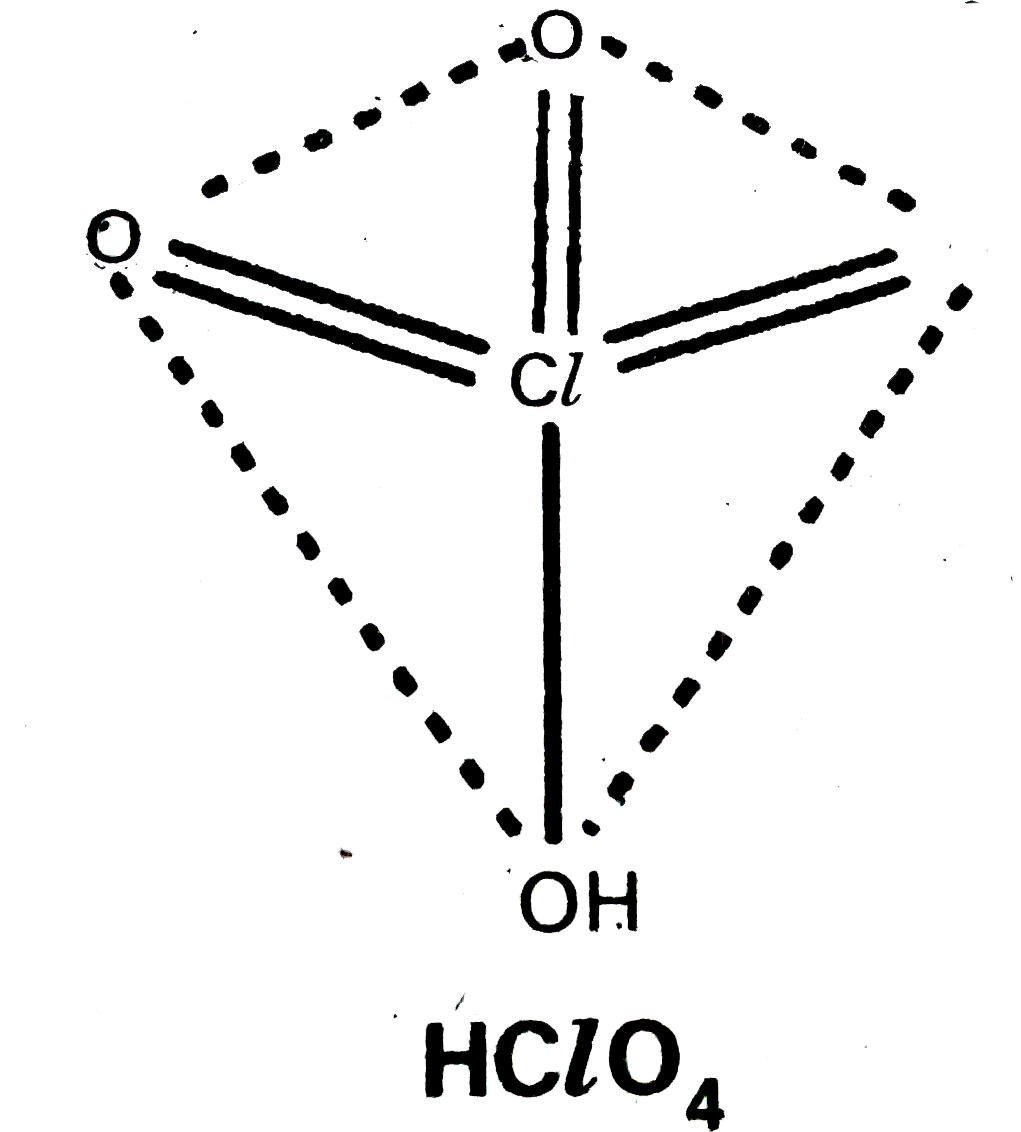
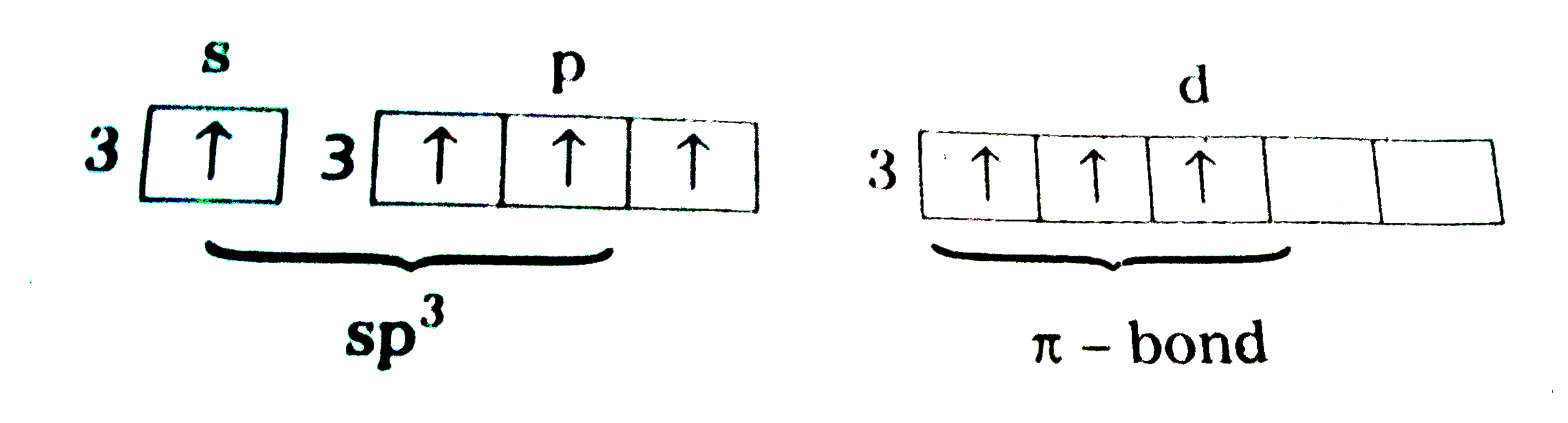Text Solution
Verified by Experts
Topper's Solved these Questions
P-BLOCK ELEMENTS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise GROUP 18 ELEMENTS (LONG ANSWER QUESTIONS)|1 VideosP-BLOCK ELEMENTS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEXT QUESTIONS|30 VideosP-BLOCK ELEMENTS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise DAM SURE LAQ - 8 Marks|2 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise DAM SURE (SAQ - 8 Marks)|15 VideosPOLYMERS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEXT QUESTIONS|6 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-P-BLOCK ELEMENTS -GROUP 17 ELEMENTS (LONG ANSWER QUESTIONS)
- How is ClF(3) prepared ? How does it react with water ? Explain its st...
Text Solution
|
- How is chlorine prepared in the laboratory ? How does it react with th...
Text Solution
|
- How is chlorine prepared in the laboratory ? How does it react with th...
Text Solution
|
- How is chlorine prepared in the laboratory ? How does it react with th...
Text Solution
|
- How is chlorine prepared in the laboratory ? How does it react with th...
Text Solution
|
- How is chlorine prepared in the laboratory ? How does it react with th...
Text Solution
|
- How is chlorine prepared in the laboratory ? How does it react with th...
Text Solution
|
- Discuss the anomalous behaviour of fluorine.
Text Solution
|
- How is chlorine prepared by electrolytic method ? Explain its reactio...
Text Solution
|
- How is chlorine prepared by electrolytic method ? Explain its reactio...
Text Solution
|
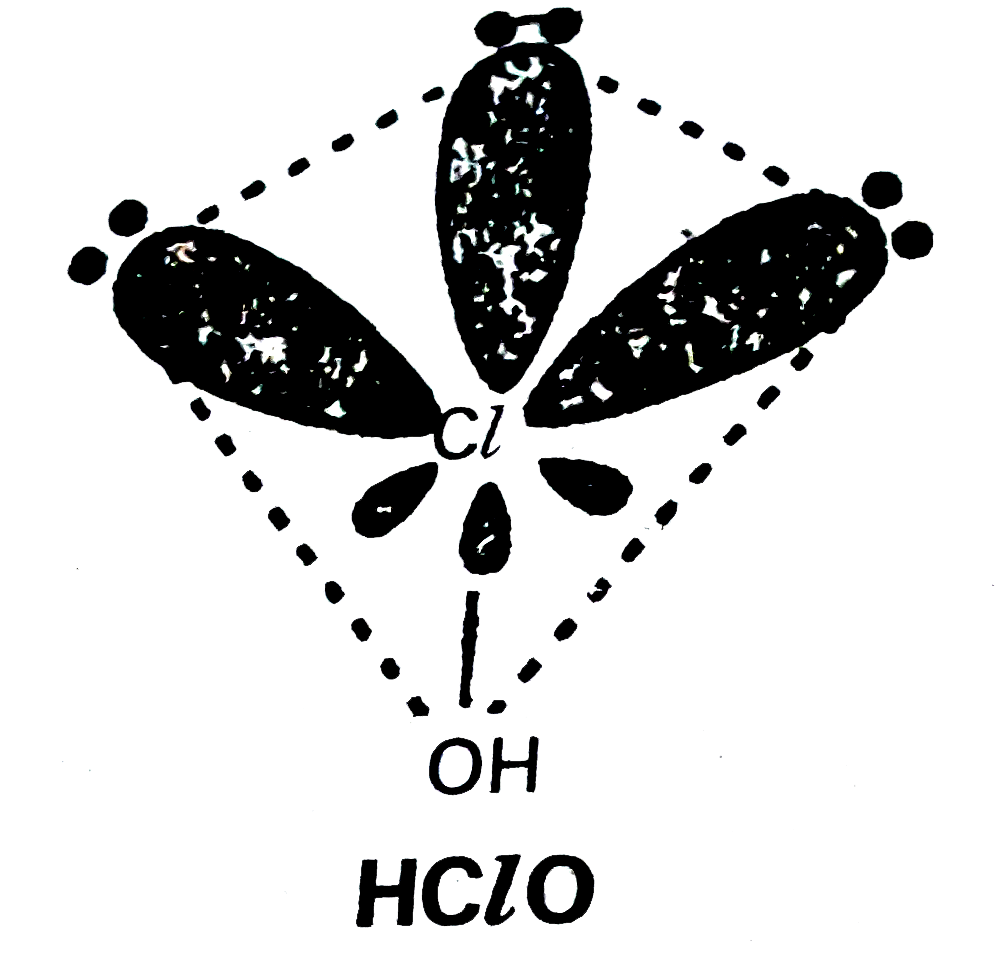
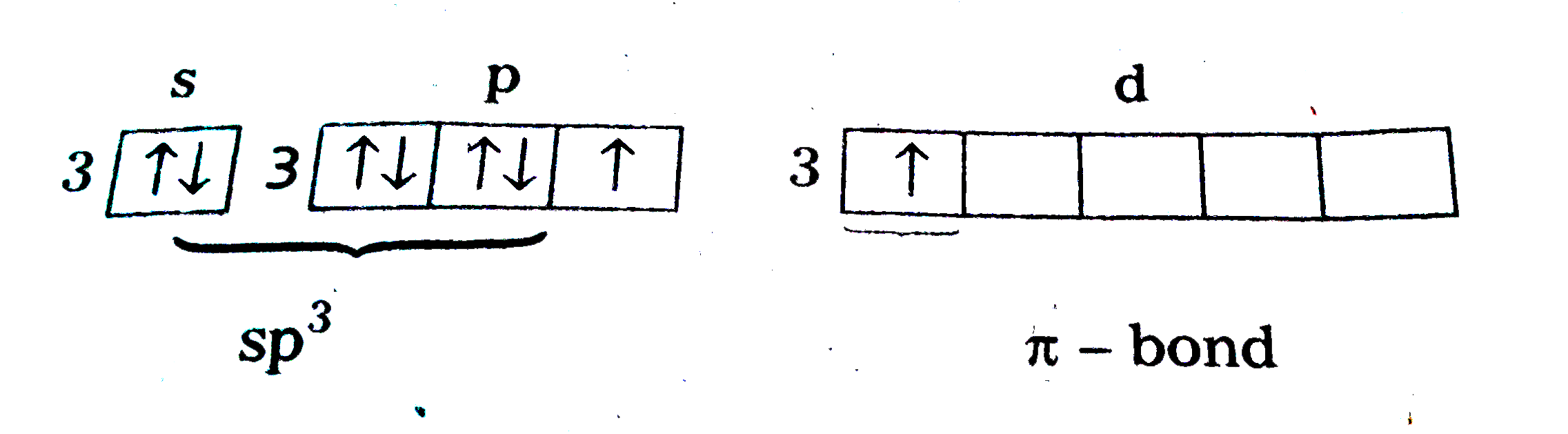
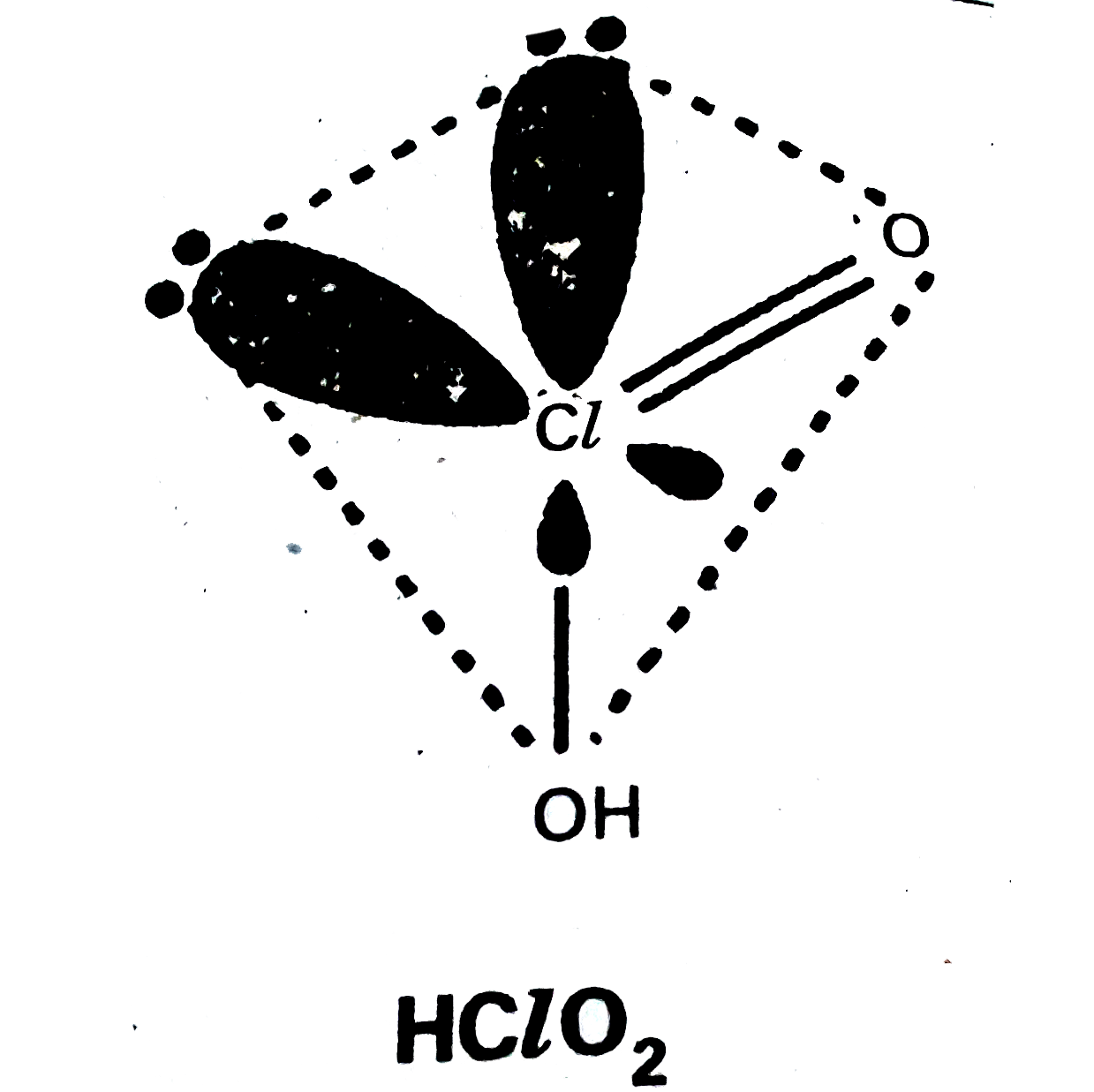
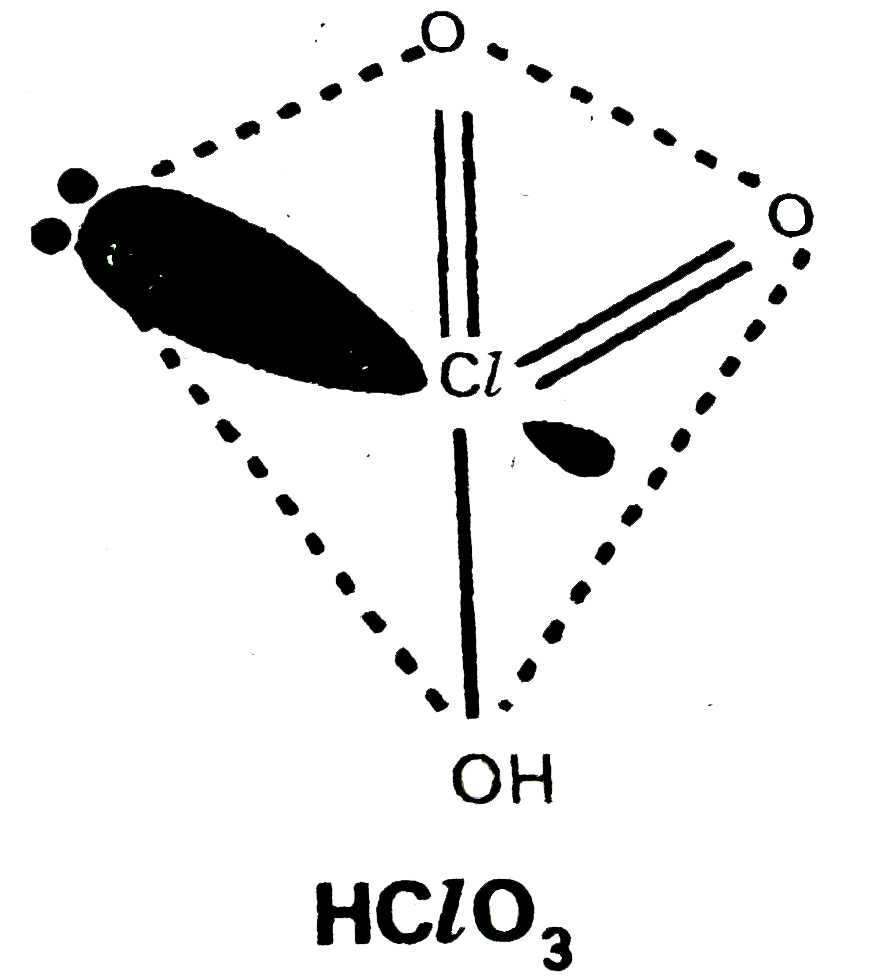
- Write the names and formulae of the oxoacids of chlorine. Explain thei...
Text Solution
|