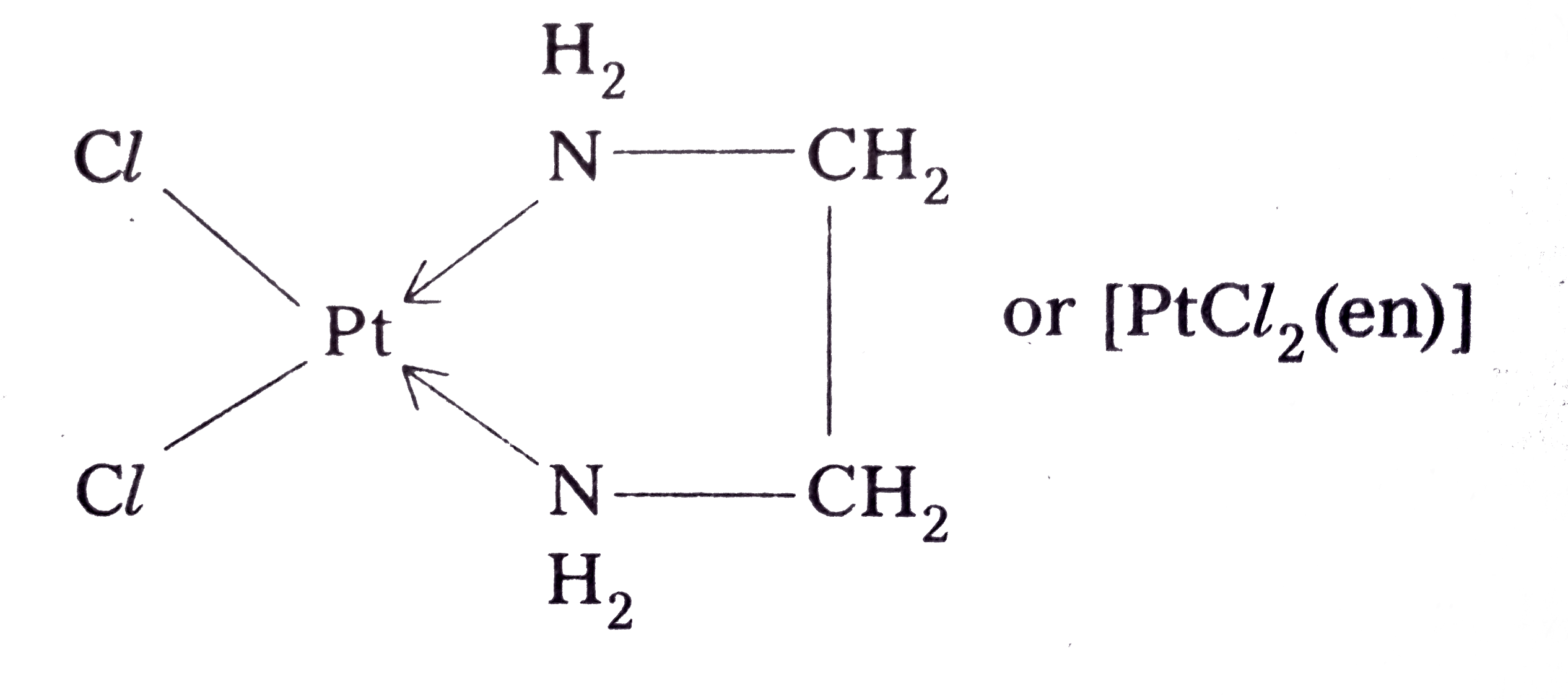
Text Solution
Verified by Experts
Topper's Solved these Questions
D AND F- BLOCK ELEMENTS AND CO-ORDINATION COMPOUNDS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise LONG ANSWER QUESTIONS|23 VideosD AND F- BLOCK ELEMENTS AND CO-ORDINATION COMPOUNDS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEXT QUESTIONS|33 VideosD AND F- BLOCK ELEMENTS AND CO-ORDINATION COMPOUNDS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise VERY SHORT ANSWER QUESTIONS|71 VideosCHEMISTRY IN EVERYDAY LIFE
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise Intex questions|5 VideosELECTROCHEMISTRY & CHEMICAL KINETICS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise DAM SURE|18 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-D AND F- BLOCK ELEMENTS AND CO-ORDINATION COMPOUNDS -SHORT ANSWER QUESTIONS
- Explain the terms polydentate ligand
Text Solution
|
- Explain the terms ambidentate ligand giving one example for each.
Text Solution
|
- What is meant by chelate effect ? Give example.
Text Solution
|
- Give the oxidation numbers of the central metal atoms in the following...
Text Solution
|
- Give the oxidation numbers of the central metal atoms in the following...
Text Solution
|
- Give the oxidation numbers of the central metal atoms in the following...
Text Solution
|
- Give the oxidation numbers of the central metal atoms in the following...
Text Solution
|
- Using IUPAC norms write the formulas for the Tetrahydroxozincate (II)
Text Solution
|
- Using IUPAC norms write the formulas for the Hexaamminecobalt (III) su...
Text Solution
|
- Using IUPAC norms write the formulas for the Potassium tetrachloropall...
Text Solution
|
- Using IUPAC norms write the formulas for the Potassium tri(oxalato) ch...
Text Solution
|
- Using IUPAC norms write the systematic names of the [Co(NH(3))(6)]Cl(3...
Text Solution
|
- Using IUPAC norms write the systematic names of the [Pt(NH(3))(2)Cl(NH...
Text Solution
|
- Using IUPAC norms write the systematic names of the [Ti(H(2)O)(6)]^(3+...
Text Solution
|
- Using IUPAC norms, write the systematic names of the [NiCl(4)]^(-2)
Text Solution
|
- Explain geometrical isomerism in Co-ordination compounds giving suitab...
Text Solution
|
- What are homoleptic and heteroleptic complexes ? Give one example for ...
Text Solution
|
- Write the characteristic properties of transition elements.
Text Solution
|
- What is lanthanoid contraction ? What are the consequences of lanthano...
Text Solution
|
- What are homoleptic and heteroleptic complexes ? Give one example for ...
Text Solution
|