Text Solution
Verified by Experts
Topper's Solved these Questions
BIOMOLECULES
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEX QUESTIONS|7 VideosBIOMOLECULES
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise DAM SURE VSAQ|18 VideosBIOMOLECULES
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise DAM SURE SAQ|10 VideosANDHRA PRADESH MARCH-2019
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise SECTION-C|5 VideosCHEMISTRY IN EVERYDAY LIFE
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise Intex questions|5 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-BIOMOLECULES -SHORT ANSWER QUESTIONS
- Explain the role of sucrose in its hydrolysis.
Text Solution
|
- Write notes on vitamins.
Text Solution
|
- What do you understand by The two strands of DNA are complementary to ...
Text Solution
|
- What are Hormones ? Give one example for each. i) Steroid Hormones ...
Text Solution
|
- Give the sources of the following vitamin and name the diseases cause...
Text Solution
|
- Explain the classification of carbohydrates.
Text Solution
|
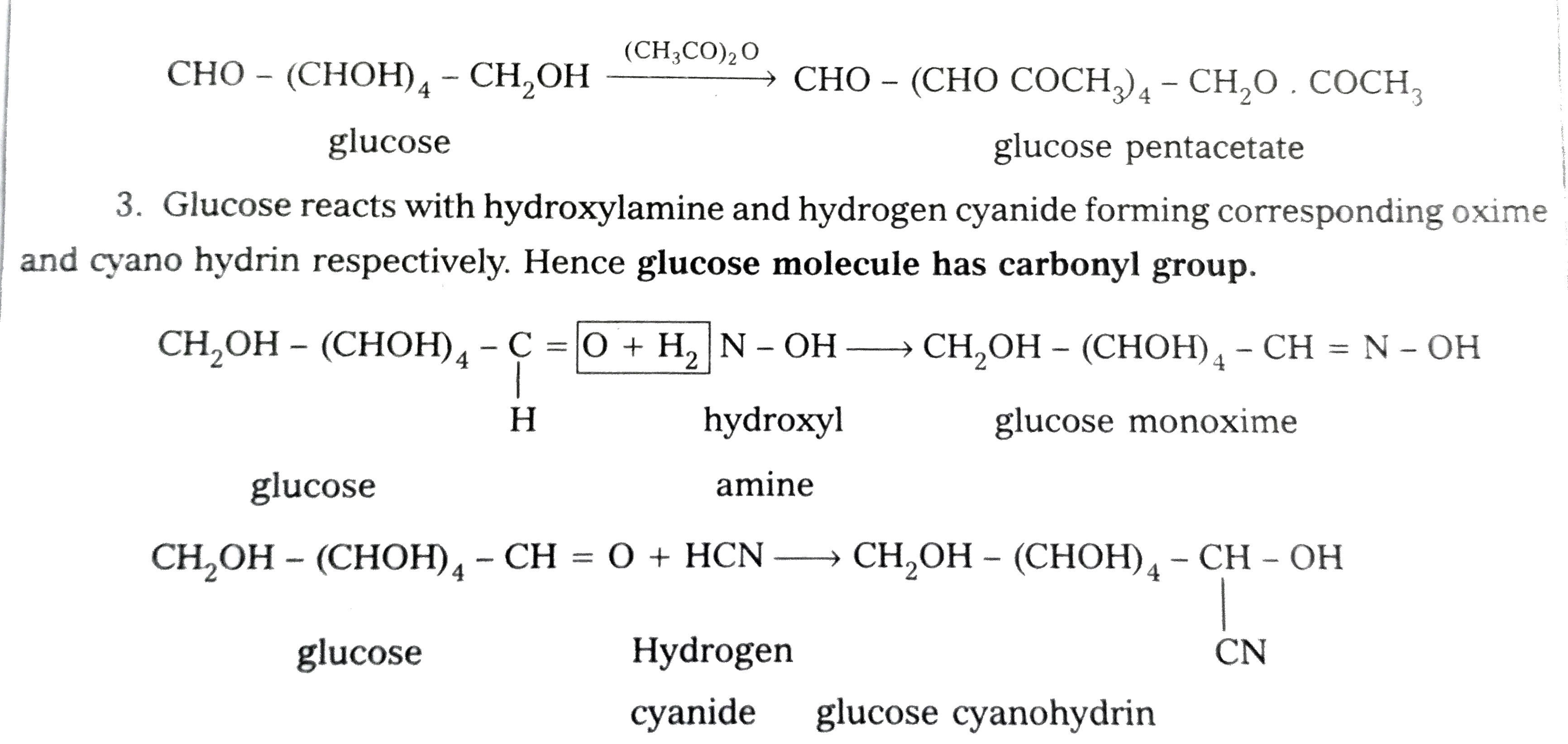
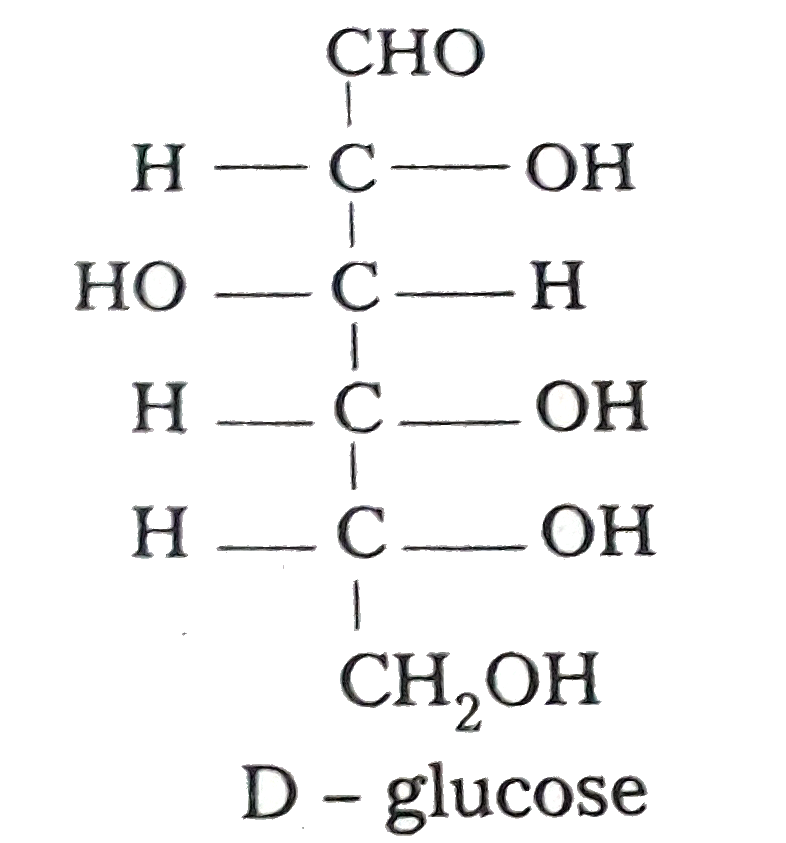
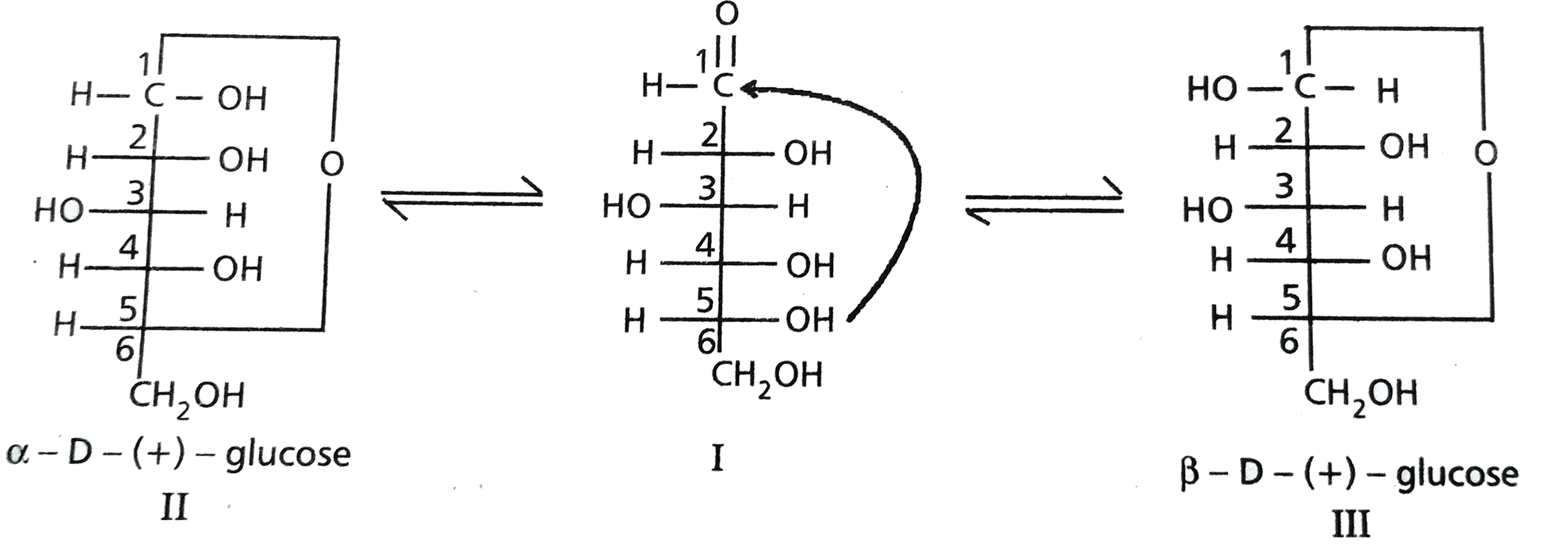
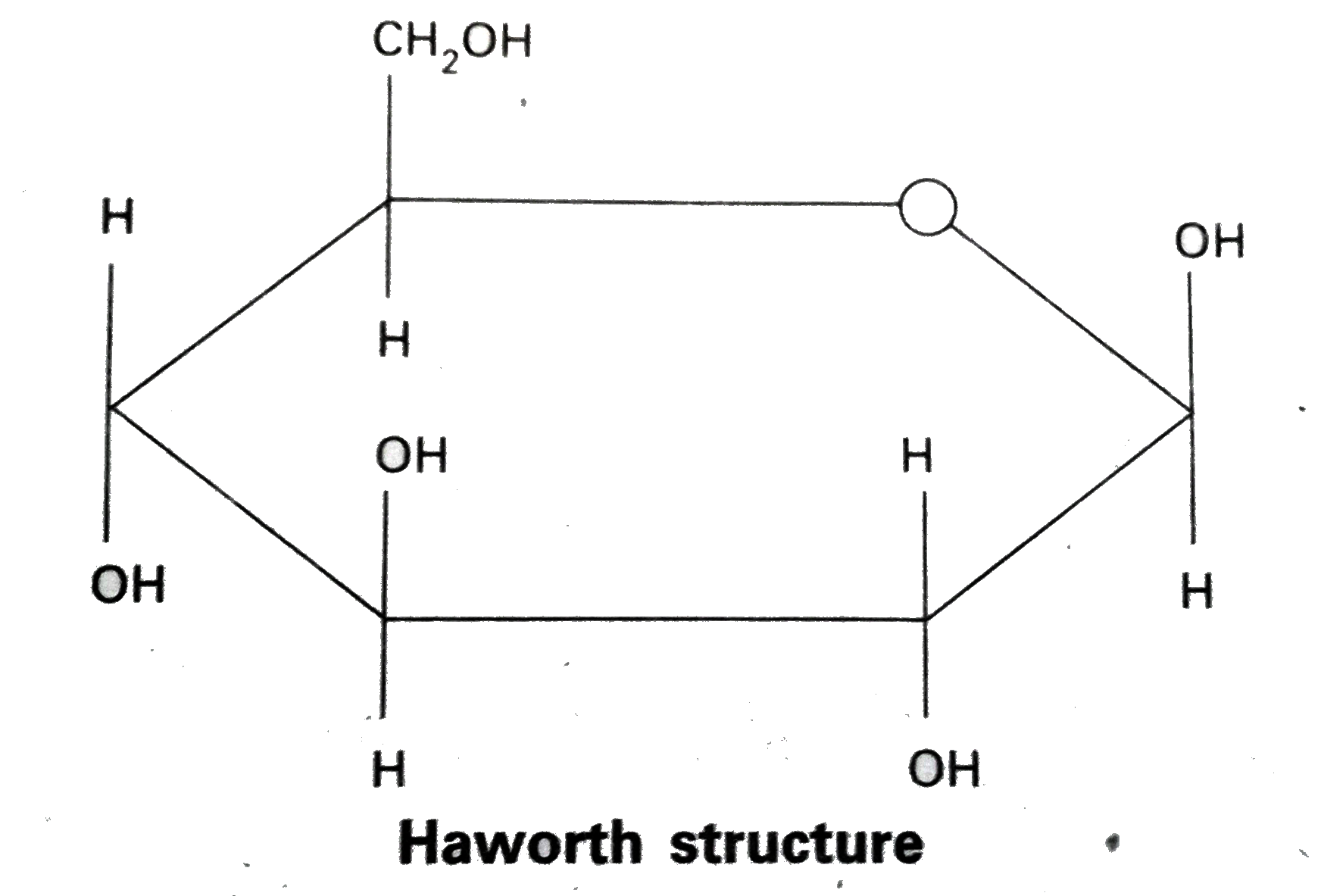
- Discuss the structure of glucose on the basis of its chemical properti...
Text Solution
|
- Write notes on fructose
Text Solution
|
- Wrire short notes on sucrose .
Text Solution
|
- Write notes on maltose
Text Solution
|
- Write notes on lactose
Text Solution
|
- Write notes on starch
Text Solution
|
- Write notes on cellulose
Text Solution
|
- Write notes on importance of carbohydrates.
Text Solution
|
- Write notes on amino acids
Text Solution
|
- Write notes on proteins.
Text Solution
|
- What are enzymes ? Give examples ?
Text Solution
|
- Write notes on vitamins.
Text Solution
|
- Explain the structures of DNA and RNA.
Text Solution
|
- Write notes on the functions of different hormones in the body.
Text Solution
|