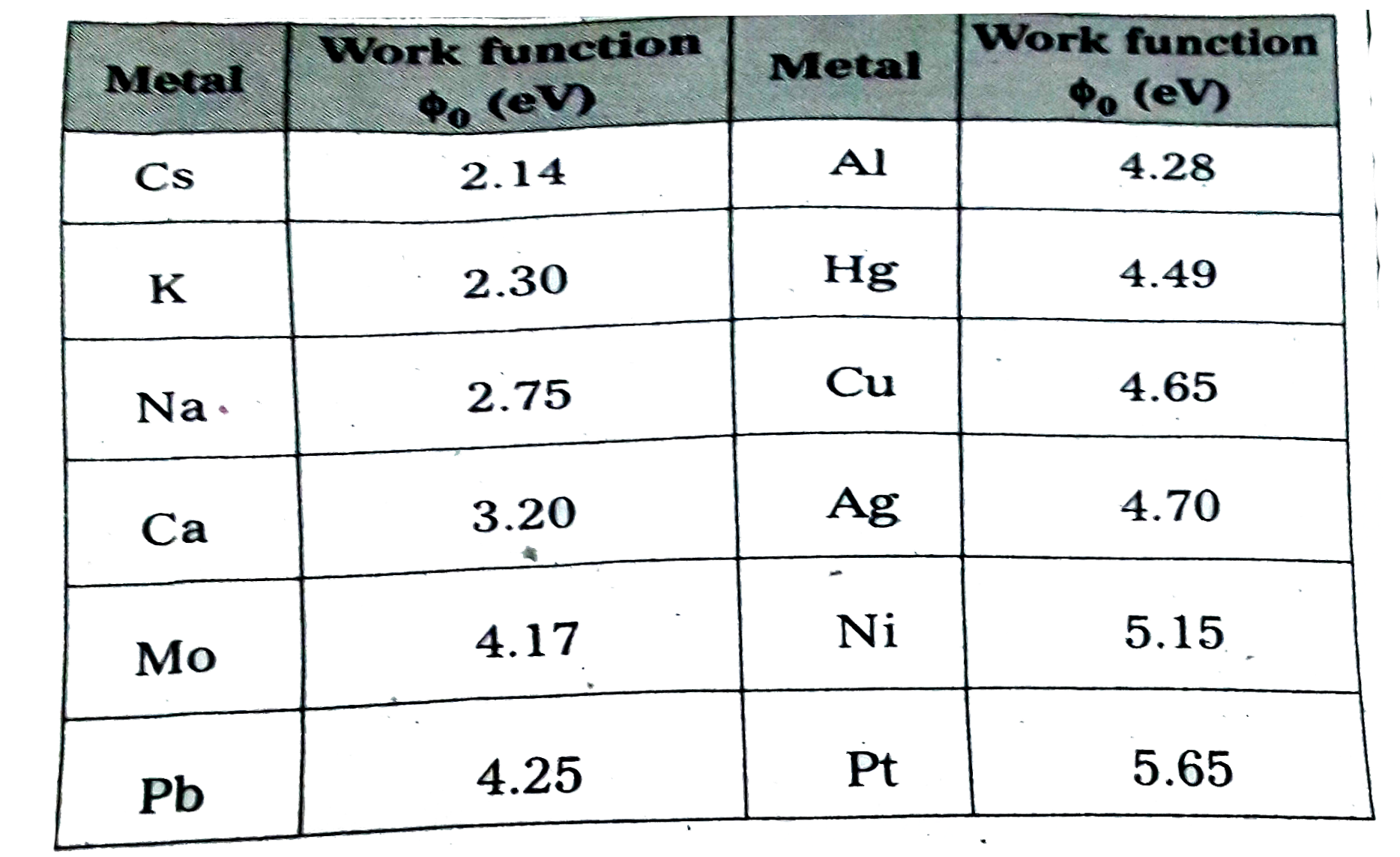
Text Solution
Verified by Experts
Topper's Solved these Questions
DUAL NATURE OF RADIATION AND MATTER
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise VERY SHORT ANSWER QUESTIONS|8 VideosDUAL NATURE OF RADIATION AND MATTER
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise SHORT ANSWER QUESTIONS|4 VideosDUAL NATURE OF RADIATION AND MATTER
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise DAM SURE (VSAQ)|2 VideosCURRENT ELECTRICITY
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise ADDITIONAL EXERCISES|22 VideosELECTRIC CHARGES AND FIELDS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise DAM SURE LAQ|1 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-DUAL NATURE OF RADIATION AND MATTER-TEXTUAL EXAMPLES
- Monochromatic light of frequency 6.0xx10^(14) Hz is produced by a lase...
Text Solution
|
- The work function of caesium is 2.14 eV . Find (a) the threshold fre...
Text Solution
|
- The wavelength of light in the visible region is about 390 nm for viol...
Text Solution
|
- The wavelength of light in the visible region is about 390 nm for viol...
Text Solution
|
- What is the de-Broglie wavelength associated with (a) an electron movi...
Text Solution
|
- An electron, an alpha-particle, and a photon have the same kinetic ene...
Text Solution
|
- A particle is moving three times as fast as an electron. The ratio of ...
Text Solution
|
- What is the de Broglie wavelength associated with an electron , acce...
Text Solution
|