A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC PHYSICS
CENGAGE PHYSICS|Exercise Maltiple correct answers type|4 VideosATOMIC PHYSICS
CENGAGE PHYSICS|Exercise Assertion-reasoning type|1 VideosATOMIC PHYSICS
CENGAGE PHYSICS|Exercise Fill In The Blanks|8 VideosALTERNATING CURRENT
CENGAGE PHYSICS|Exercise QUESTION BANK|65 VideosATOMS
CENGAGE PHYSICS|Exercise QUESTION BANK|40 Videos
Similar Questions
Explore conceptually related problems
CENGAGE PHYSICS-ATOMIC PHYSICS-Single correct answer type
- Consider the spectral line resulting from the transition n = 2 rarr n...
Text Solution
|
- The X-ray beam coming from an X-ray tube
Text Solution
|
- The K(alpha) X-ray emission line of lungsten accurs at lambda = 0.021 ...
Text Solution
|
- As per Bohr model , the minimum energy (in eV) required to remove elec...
Text Solution
|
- X- rays are produced in an X- rays tube operating at a given accelerat...
Text Solution
|
- Imagine an atom made up of a proton and a hypothetical particle of do...
Text Solution
|
- The electron in a hydrogen atom makes a transition from an excited sta...
Text Solution
|
- Electrons with energy 80 keV are incident on the tungsten target of an...
Text Solution
|
- The transition from the state n = 4 to n = 3 in a hydrogen - like ato...
Text Solution
|
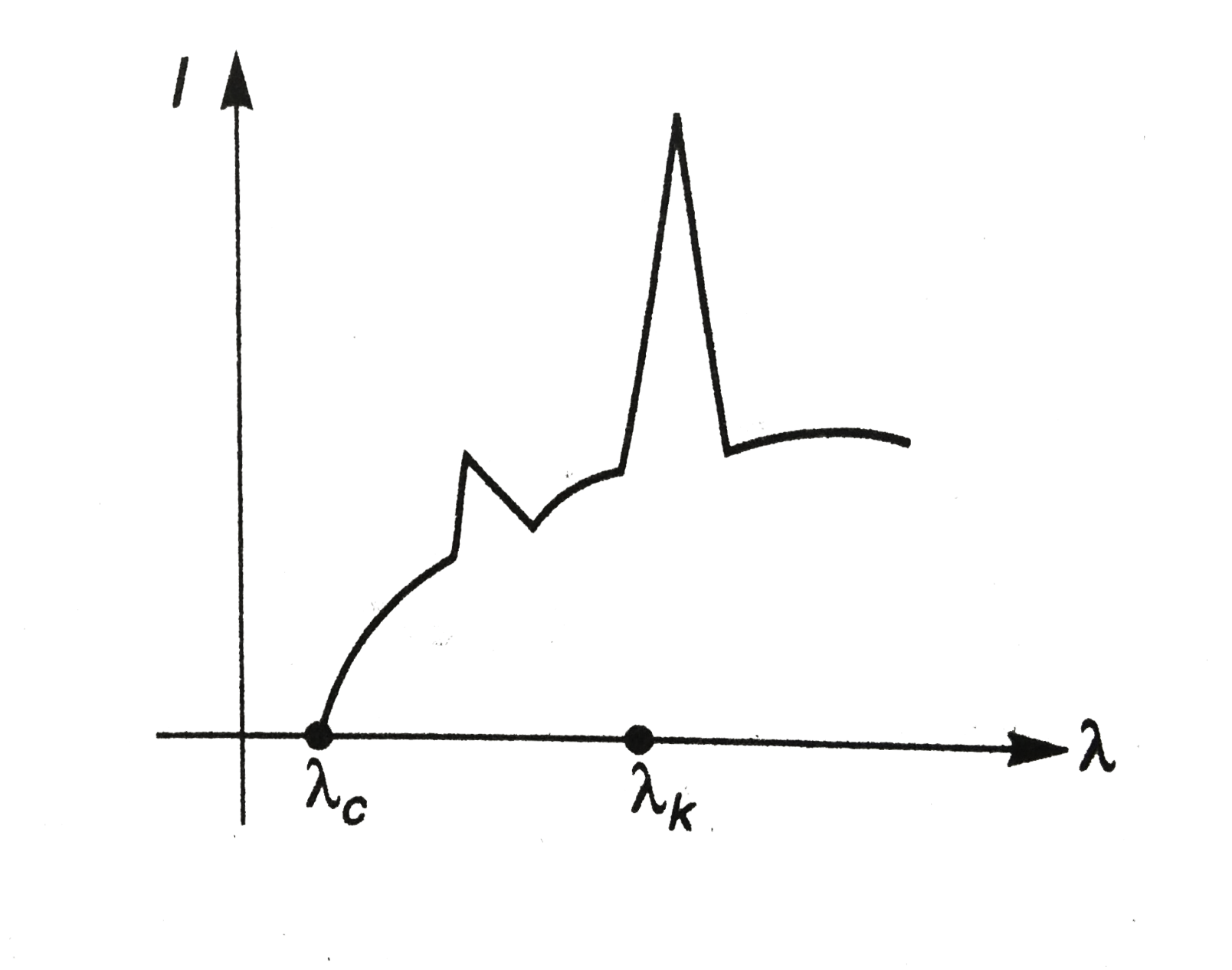
- The intensity of X-rays form a Coolidge tube is plotted against wavel...
Text Solution
|
- The potential difference applied to an X-ray tube is 5k V and the curr...
Text Solution
|
- A Hydrogen atom and Li^(++) ion are both in the second excited state ....
Text Solution
|
- The electric potential between a proton and as electron is given by V=...
Text Solution
|
- If the atom(100)Fm^(257) follows the Bohr model the radius of (100)Fm^...
Text Solution
|
- k(a) wavelength emitted by an atom of atomic number Z= 11 is lambda f...
Text Solution
|
- A photon collides with a stationary hydrogen atom in ground state inel...
Text Solution
|
- The largest wavelength in the ultraviolet region of the hydrogen spect...
Text Solution
|
- Electrons with de- Broglie wavelength lambda fall on the target in an ...
Text Solution
|
- Which one of the following statement is WRONG in the context of X- ray...
Text Solution
|
- The wavelength of the first spectral line in the Balmer series of hydr...
Text Solution
|