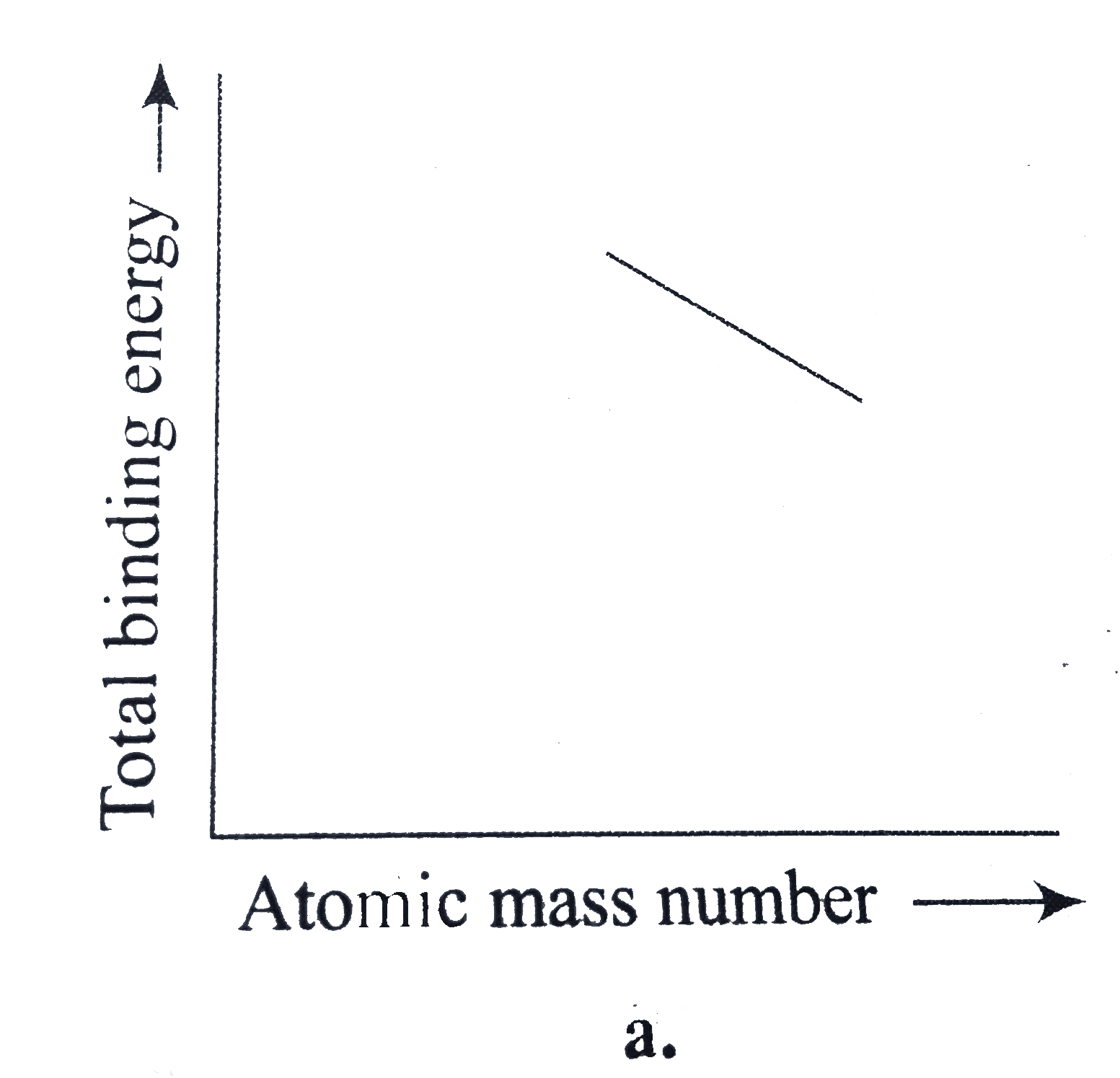
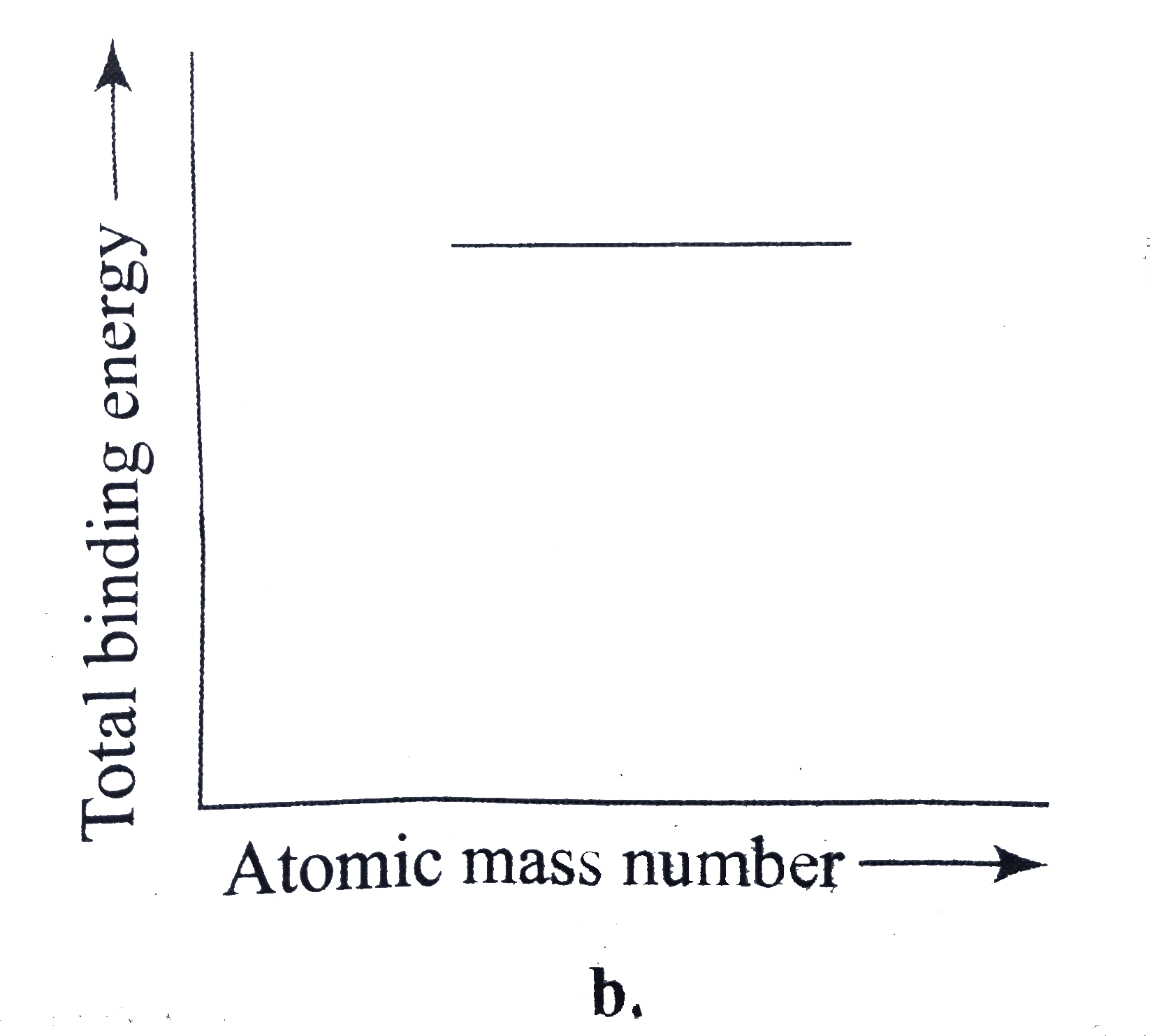
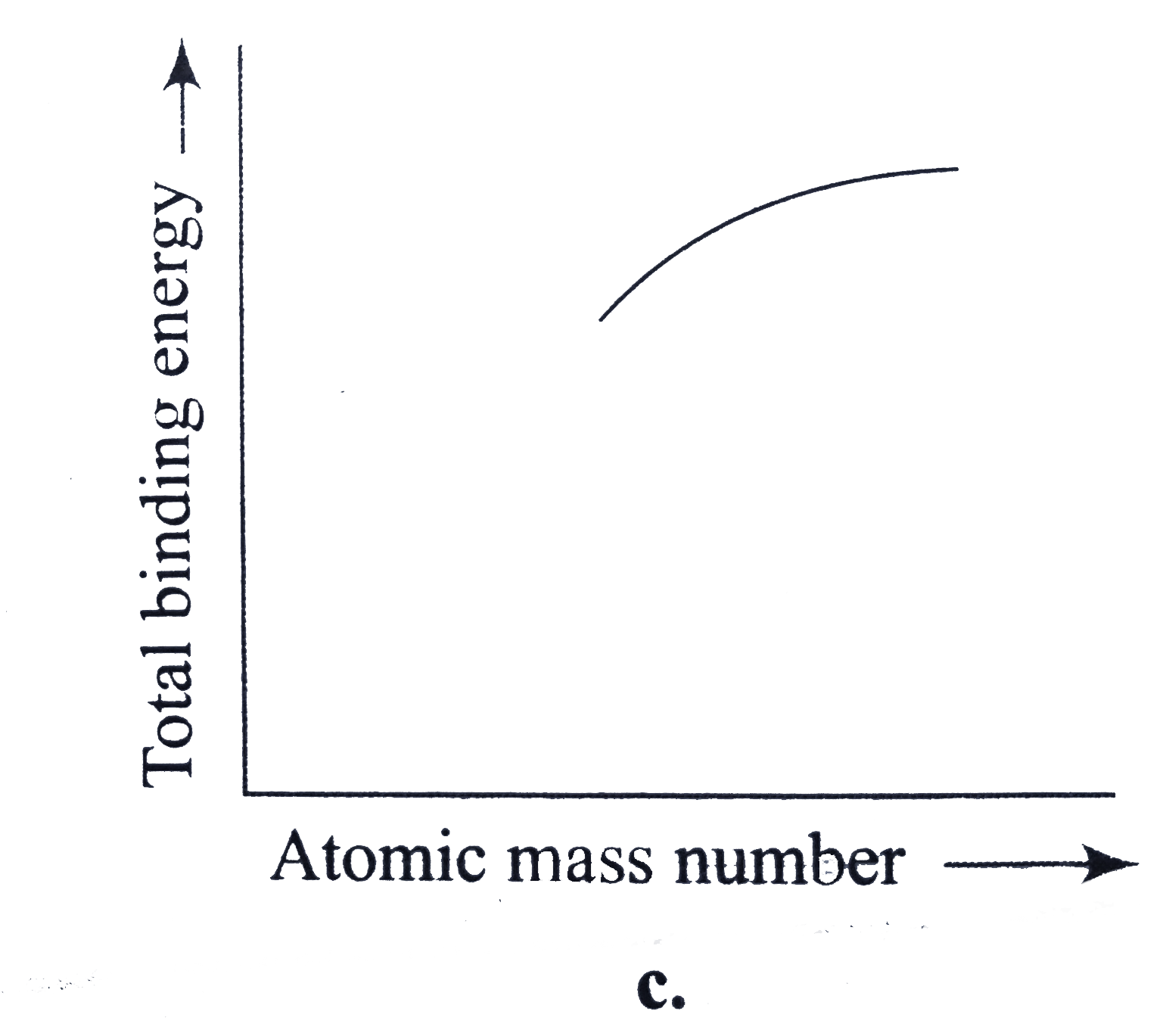
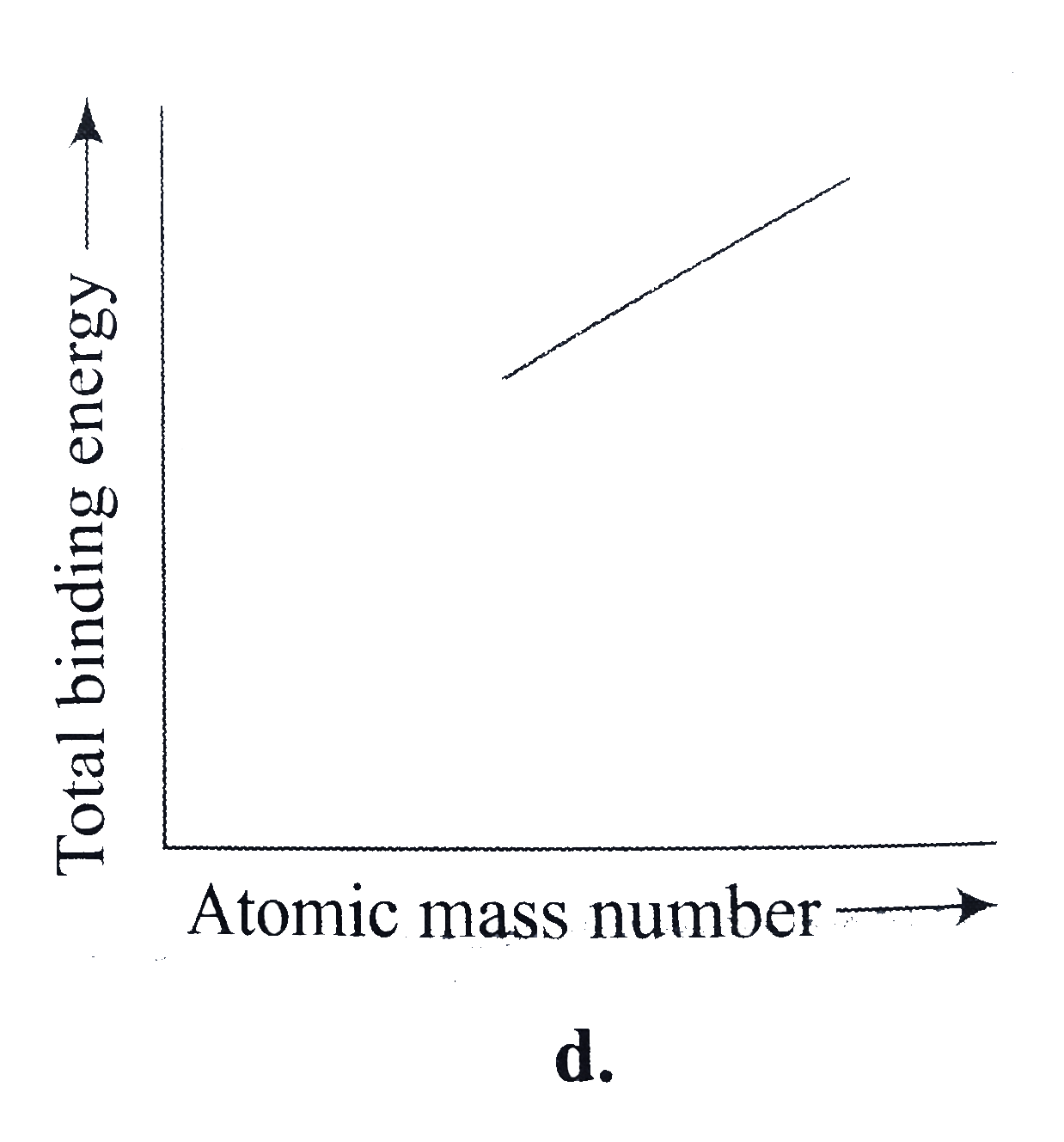
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
NUCLEAR PHYSICS
CENGAGE PHYSICS|Exercise Integer|6 VideosNUCLEAR PHYSICS
CENGAGE PHYSICS|Exercise Fill In The Blanks|5 VideosNUCLEAR PHYSICS
CENGAGE PHYSICS|Exercise Single Correct Option|182 VideosMoving charges and magnetism
CENGAGE PHYSICS|Exercise Question Bank|20 VideosNUCLEI
CENGAGE PHYSICS|Exercise QUESTION BANK|59 Videos
Similar Questions
Explore conceptually related problems
CENGAGE PHYSICS-NUCLEAR PHYSICS-Linked Comprehension
- Many unstable nuclie can decay spontaneously to a nucleus of lower mas...
Text Solution
|
- Many unstable nuclie can decay spontaneously to a nucleus of lower mas...
Text Solution
|
- All nuclei consist of two types of particles- protaon and neutrons. Nu...
Text Solution
|
- All nuclei consist of two types of particles- protaon and neutrons. Nu...
Text Solution
|
- All nuclei consist of two types of particles- protaon and neutrons. Nu...
Text Solution
|
- The compound unstabel nucleus .(92)^(236)U often decays in accordance ...
Text Solution
|
- The compound unstabel nucleus .(92)^(236)U often decays in accordance ...
Text Solution
|
- The compound unstabel nucleus .(92)^(236)U often decays in accordance ...
Text Solution
|
- The compound unstabel nucleus .(92)^(236)U often decays in accordance ...
Text Solution
|
- A beam of alpha paricles is incident on a target of lead. A particular...
Text Solution
|
- A beam of alpha paricles is incident on a target of lead. A particular...
Text Solution
|
- A beam of alpha paricles is incident on a target of lead. A particular...
Text Solution
|
- A nucleus kept at rest in free space, brakes up into smaller nuclei of...
Text Solution
|
- A nucleus kept at rest in free space, brakes up into smaller nuclei of...
Text Solution
|
- A nucleus kept at rest in free space, brakes up into smaller nuclei of...
Text Solution
|
- Nuceli A and B convert into a stable nucleus C. Nucleus A is converted...
Text Solution
|
- The half-life of a radioactive nuclide is 20 hours. What fraction of o...
Text Solution
|
- A radioactive sample has 8.0xx10^(18) active nuclei at a certain insta...
Text Solution
|
- A radioactive sample decays with an average life of 20 ms. A capacitor...
Text Solution
|
- A radioactive sample decays through two different decay processes alph...
Text Solution
|