Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY|Exercise Ex 2 .2 Subjective (Molecular Orbital Theory)|4 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY|Exercise Ex 2 .2 Objective|34 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY|Exercise Solved Examples|36 VideosATOMIC STRUCTURE
CENGAGE CHEMISTRY|Exercise Concept Applicationexercise(4.3)|19 VideosCHEMICAL EQUILIBRIUM
CENGAGE CHEMISTRY|Exercise Archives (Subjective)|11 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-CHEMICAL BONDING AND MOLECULAR STRUCTURE-Ex 2 .2 Subjective (Intermolecular Forces And H-Bonding)
- Write a Lewis structure for "CC"I(2)F(2) one of the compounds indicate...
Text Solution
|
- Write Lewis structure for the following (a) Ethene (C(2)H(4)) the mo...
Text Solution
|
- The dipole moment of LiH is 1.964 xx 10^(-29)Cm and interatomic distan...
Text Solution
|
- Predict whether each of the following molecule has a dipole momnet (...
Text Solution
|
- The dipole moment of KCI is 3.36 xx 10^(-29)Cm The interatomic distanc...
Text Solution
|
- Account of the following observations (a) Ammonium salts are more so...
Text Solution
|
- State whether the following are ionic or covalent (a) CaH(2) (b) MgO...
Text Solution
|

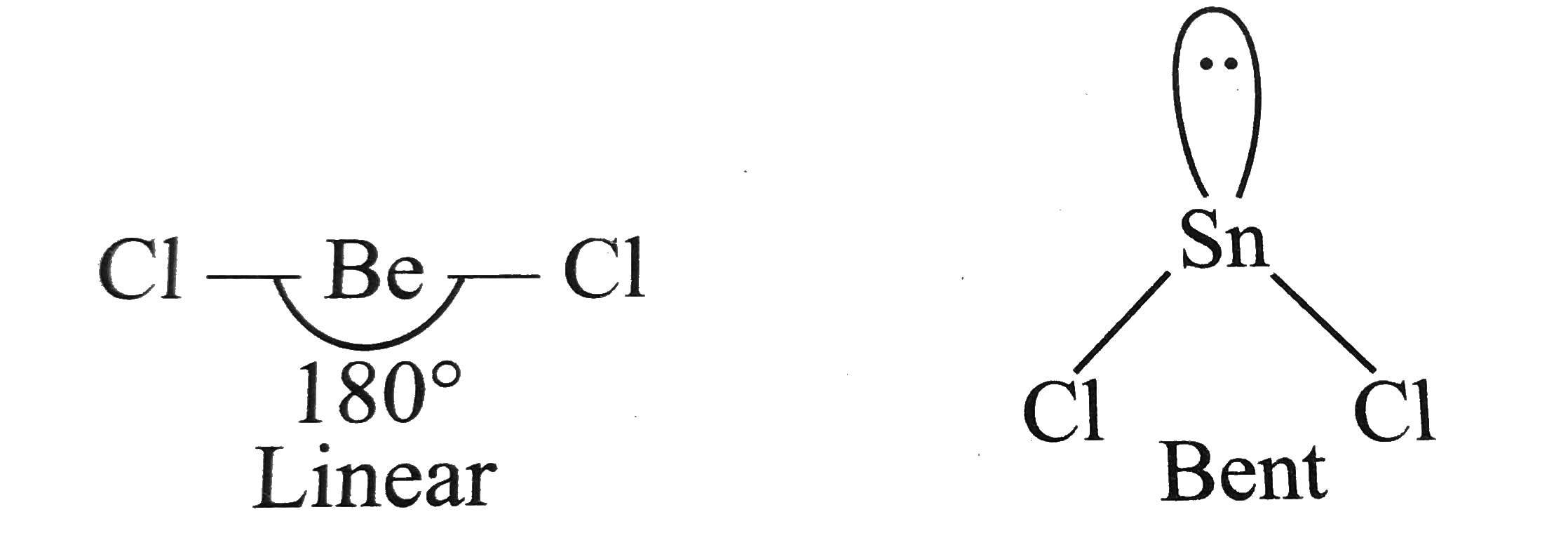 .
.