Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY|Exercise Ex 2 .2 Objective|34 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY|Exercise Exercises Linked Comprehension|43 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY|Exercise Ex 2 .2 Subjective (Intermolecular Forces And H-Bonding)|7 VideosATOMIC STRUCTURE
CENGAGE CHEMISTRY|Exercise Concept Applicationexercise(4.3)|19 VideosCHEMICAL EQUILIBRIUM
CENGAGE CHEMISTRY|Exercise Archives (Subjective)|11 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-CHEMICAL BONDING AND MOLECULAR STRUCTURE-Ex 2 .2 Subjective (Molecular Orbital Theory)
- Identify which of them are polar and non-polar (a) HF (b) BeCI(2) (c...
Text Solution
|
- Give reasons for the following (a) PF(5) is know but NF(3) is not ...
Text Solution
|
- Give reasons for the following (a) CO(2) has no dipole moment but SO...
Text Solution
|
- Indicate wheter the following pairs of elements form ionic or covalent...
Text Solution
|
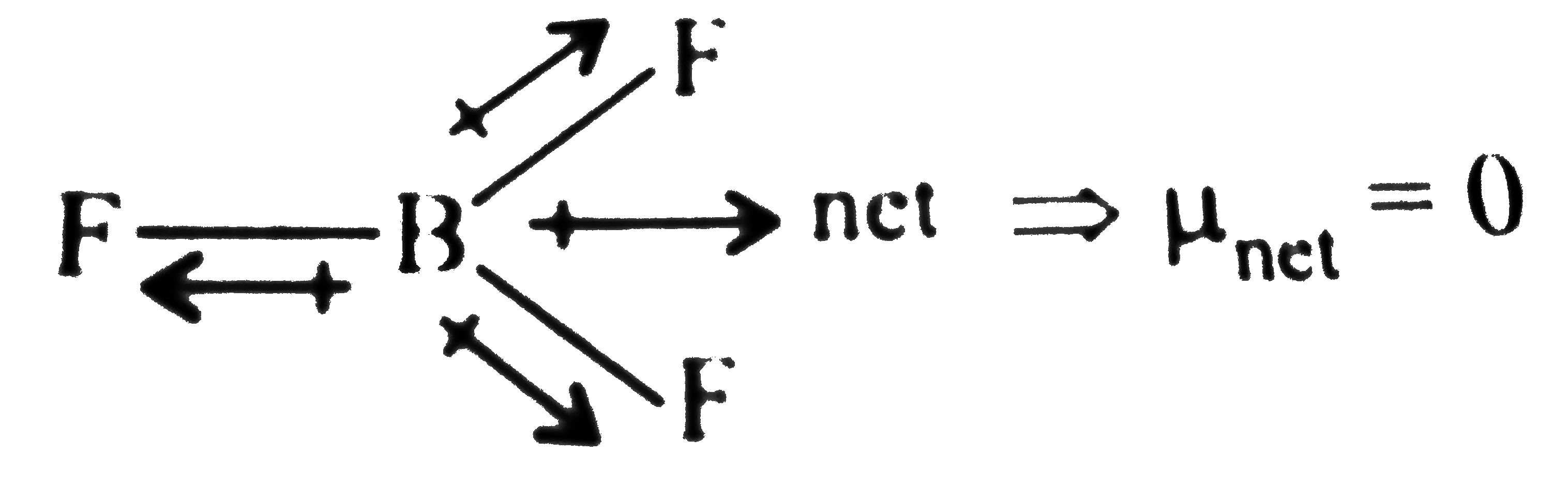
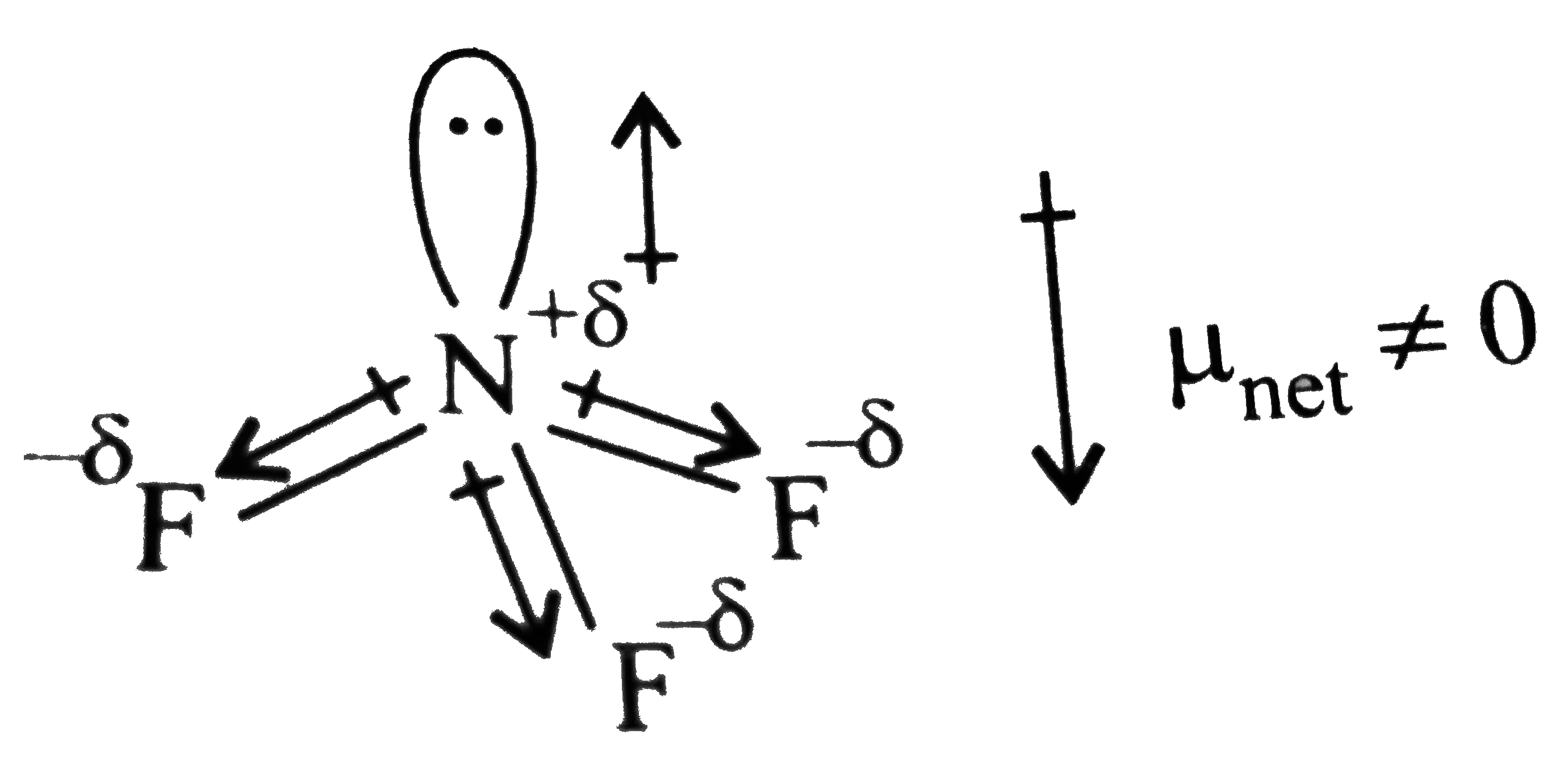
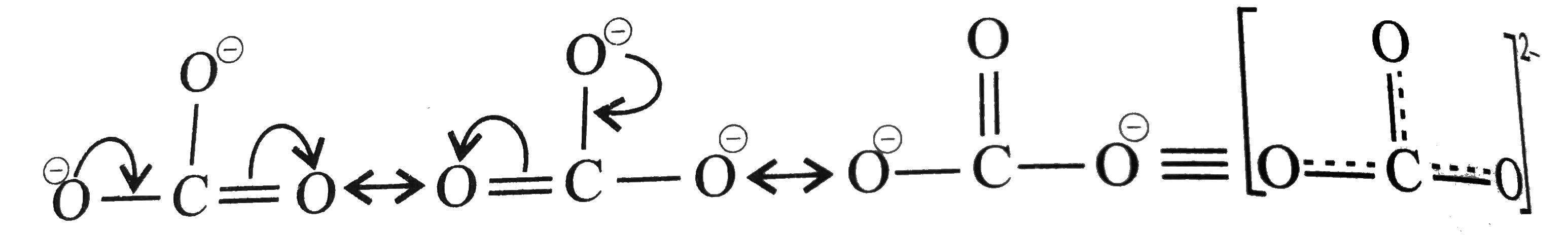 .
.