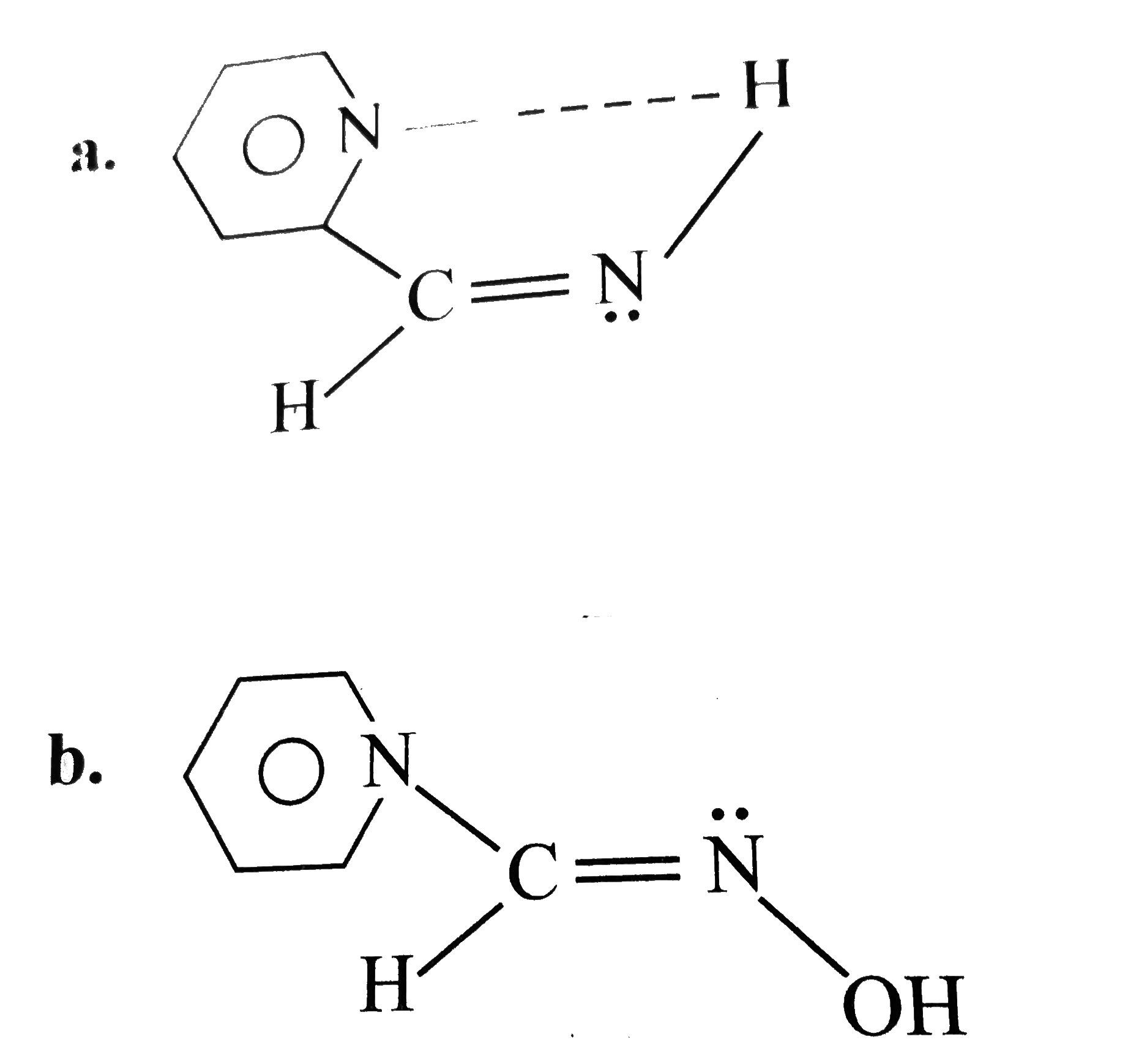
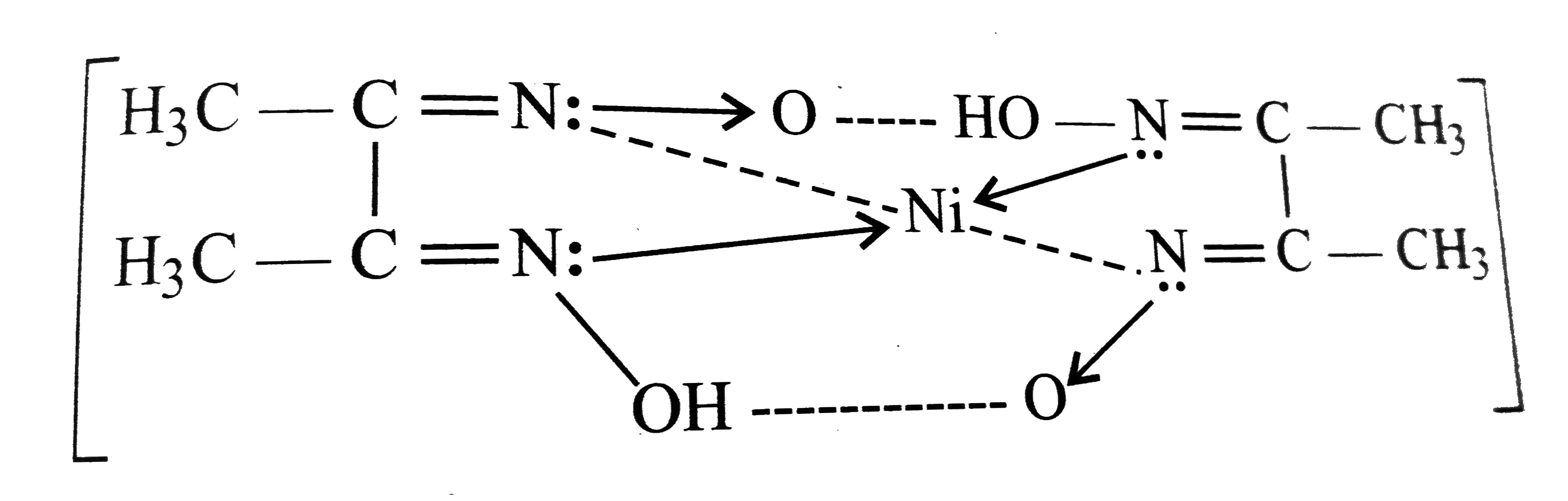
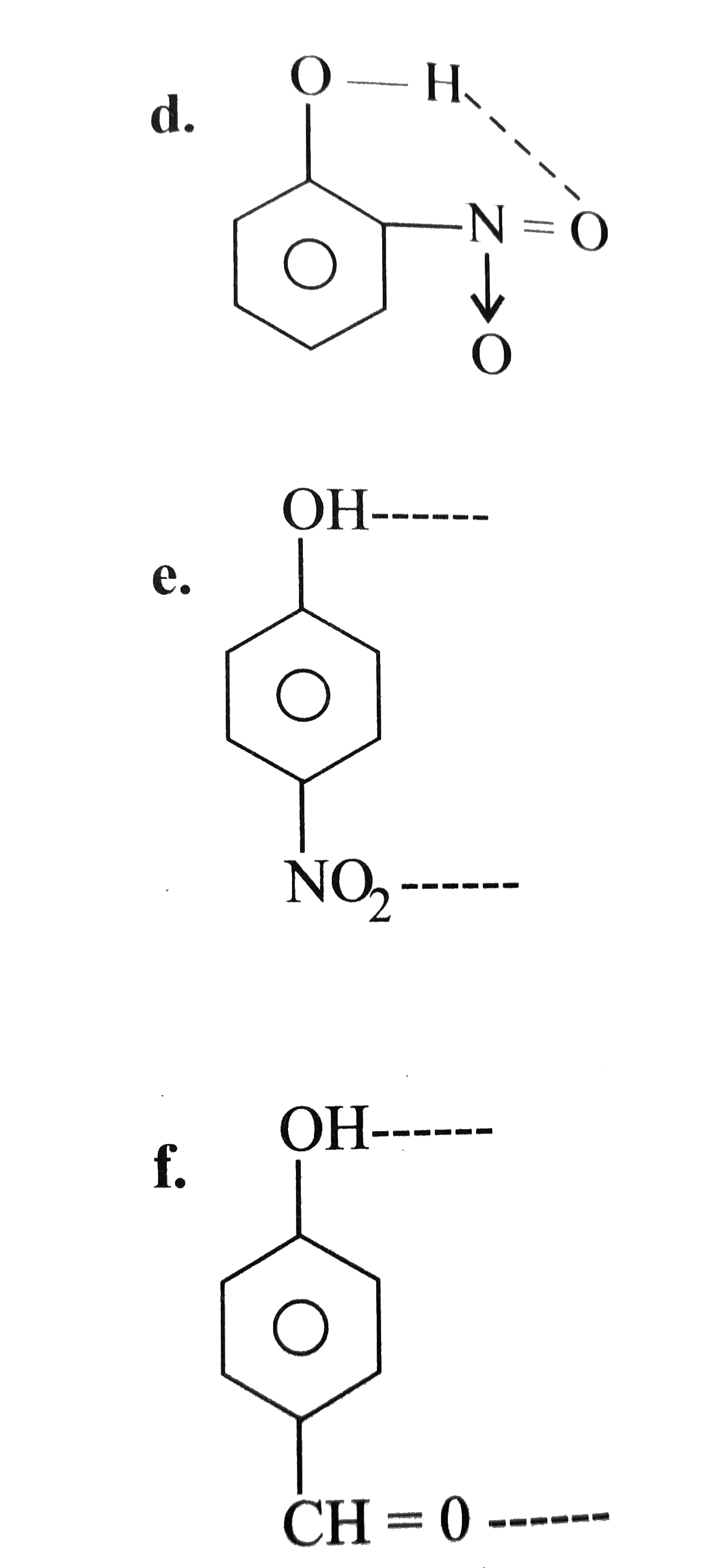
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY|Exercise Exercises Multiple Correct(Chemical Bonding )|7 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY|Exercise Exercises Multiple Correct (Dipole Moment)|3 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY|Exercise Ex 2 .2 Objective|34 VideosATOMIC STRUCTURE
CENGAGE CHEMISTRY|Exercise Concept Applicationexercise(4.3)|19 VideosCHEMICAL EQUILIBRIUM
CENGAGE CHEMISTRY|Exercise Archives (Subjective)|11 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-CHEMICAL BONDING AND MOLECULAR STRUCTURE-Exercises Linked Comprehension
- The HF(2)^(Θ) ion solid state and in liquid HF but not in the dilute a...
Text Solution
|
- The HF(2)^(Θ) ion solid state and in liquid HF but not in the dilute a...
Text Solution
|
- The HF(2)^(Θ) ion solid state and in liquid HF but not in the dilute a...
Text Solution
|
- The HF(2)^(Θ) ion solid state and in liquid HF but not in the dilute a...
Text Solution
|
- Valence-bond theory is one of the two quantum mechanical approaches th...
Text Solution
|
- Valence-bond theory is one of the two quantum mechanical approaches th...
Text Solution
|
- Valence-bond theory is one of the two quantum mechanical approaches th...
Text Solution
|
- Valence-bond theory is one of the two quantum mechanical approaches th...
Text Solution
|
- Valence-bond theory is one of the two quantum mechanical approaches th...
Text Solution
|
- According to the moleular orbital theory, all atomic orbitals combine ...
Text Solution
|
- According to the moleular orbital theory, all atomic orbitals combine ...
Text Solution
|
- According to the moleular orbital theory, all atomic orbitals combine ...
Text Solution
|
- Most of the polyatomic molecules except a few such as CO(2) and CS(2) ...
Text Solution
|
- Most of the polyatomic molecules except a few such as CO(2) and CS(2) ...
Text Solution
|
- Most of the polyatomic molecules except a few such as CO(2) and CS(2) ...
Text Solution
|
- MO' s are formed by the overlap of A'O s Two AO's combine to form two ...
Text Solution
|
- MO' s are formed by the overlap of A'O s Two AO's combine to form two ...
Text Solution
|
- MO' s are formed by the overlap of A'O s Two AO's combine to form two ...
Text Solution
|
- MO' s are formed by the overlap of A'O s Two AO's combine to form two ...
Text Solution
|
- MO' s are formed by the overlap of A'O s Two AO's combine to form two ...
Text Solution
|