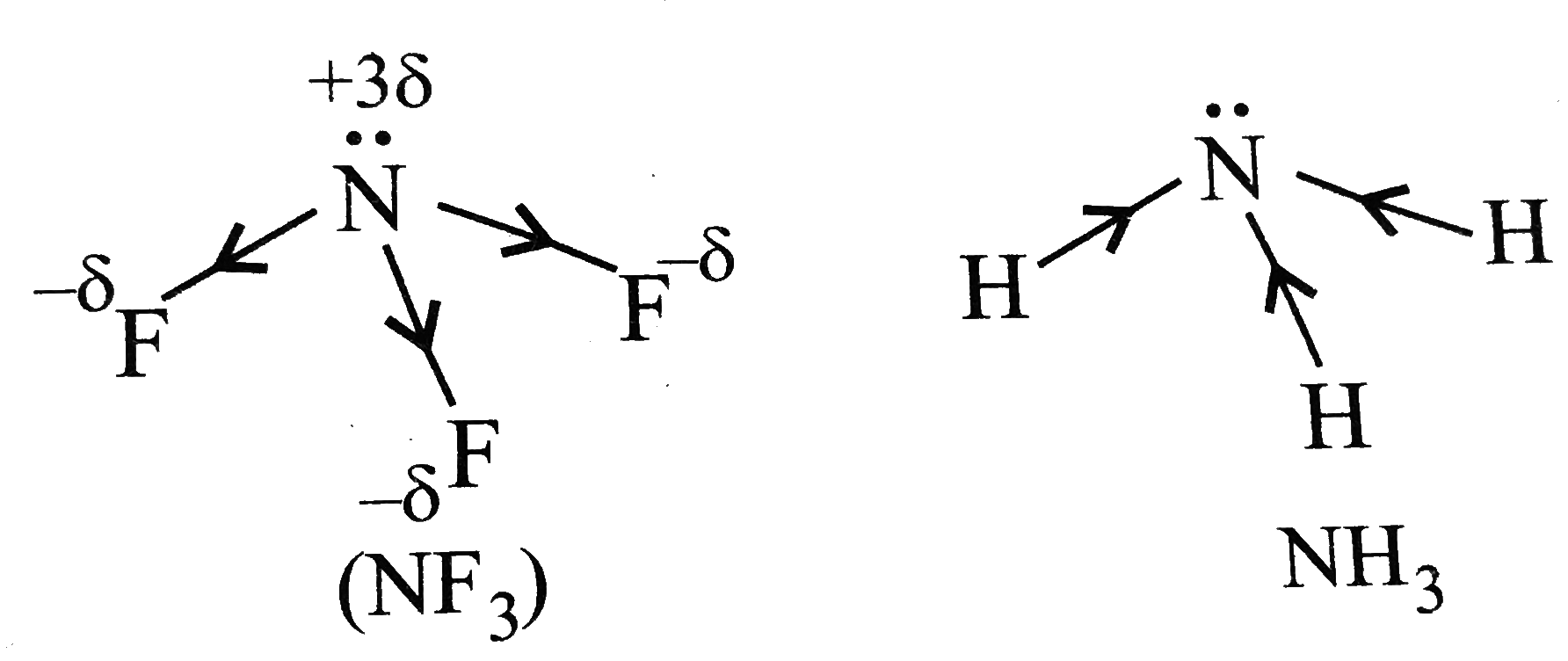
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY|Exercise Exercises Single Correct (Chemical Bonding)|46 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY|Exercise Exercises Single Correct (Dipole Moment)|16 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY|Exercise Exercises Multiple Correct(Molecular Orbitaltheory (Mot))|10 VideosATOMIC STRUCTURE
CENGAGE CHEMISTRY|Exercise Concept Applicationexercise(4.3)|19 VideosCHEMICAL EQUILIBRIUM
CENGAGE CHEMISTRY|Exercise Archives (Subjective)|11 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-CHEMICAL BONDING AND MOLECULAR STRUCTURE-Exercises Multiple Correct (Miscellaneous)
- Select corrcet orders for corresponding property as indicated in brack...
Text Solution
|
- Which one or more among the following involve (s) (pi -dpi) bonding ? ...
Text Solution
|
- Paramagnetic pairs (s) among the following is (are) .
Text Solution
|
- Which of the following orders are correct for property indicated in br...
Text Solution
|
- The first element of groups 13-16 differ rest of the elements This is ...
Text Solution
|
- Select the correct satements .
Text Solution
|
- Write vitriol is not isomorphous with .
Text Solution
|
- The stability of ions of Ge Sn and Pb will be in the order .
Text Solution
|
- Select the correct satements (s) .
Text Solution
|
- Which of the following are true ?.
Text Solution
|