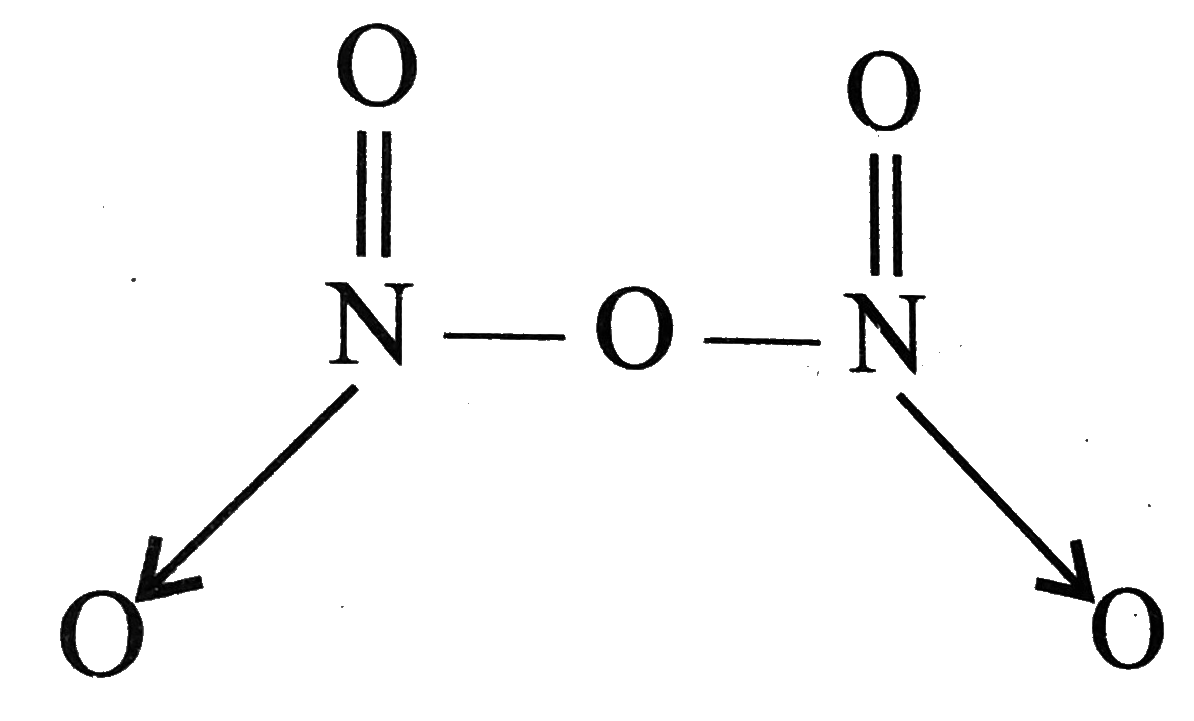
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY|Exercise Exercises Single Correct (Dipole Moment)|16 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY|Exercise Exercises Single Correct (Hybridisation)|33 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY|Exercise Exercises Multiple Correct (Miscellaneous)|10 VideosATOMIC STRUCTURE
CENGAGE CHEMISTRY|Exercise Concept Applicationexercise(4.3)|19 VideosCHEMICAL EQUILIBRIUM
CENGAGE CHEMISTRY|Exercise Archives (Subjective)|11 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-CHEMICAL BONDING AND MOLECULAR STRUCTURE-Exercises Single Correct (Chemical Bonding)
- Which species has the maximum number of lone pair of electrons on the ...
Text Solution
|
- Among the following electrons-deficient compound is .
Text Solution
|
- Which of the following does not follow the octet rule ? .
Text Solution
|
- Which of the following does not have coordinate bonds ? .
Text Solution
|
- Which of the following bonds is the strongest ? .
Text Solution
|
- When two atoms combine to form a molecule .
Text Solution
|
- Most favourable conditions for inoic bonding are .
Text Solution
|
- Which of the following is not a correct statement ? .
Text Solution
|
- Element A has three electrons in the outermost orbit and B has six ele...
Text Solution
|
- The pair of elements which form ionic bond is .
Text Solution
|
- Lattice energy of an ionic compound depedns upon :
Text Solution
|
- The bonds present in N(2)O(5) are .
Text Solution
|
- Which of the following statement is correct for CO ?
Text Solution
|
- Which of the following statemwnt regarding valence bond theory (VBT) i...
Text Solution
|
- Correct statement about VBT is .
Text Solution
|
- The strength of bonds formed by overlapping of atomic orbitals is in t...
Text Solution
|
- The nodal plane in the pi-bond of ethene is located in:
Text Solution
|
- Which of the following statement is wrong ? .
Text Solution
|
- Which of the following is a positive overlap which leads to bonding ? ...
Text Solution
|
- Which of the following is a zero overlap which leads to non-bonding?
Text Solution
|