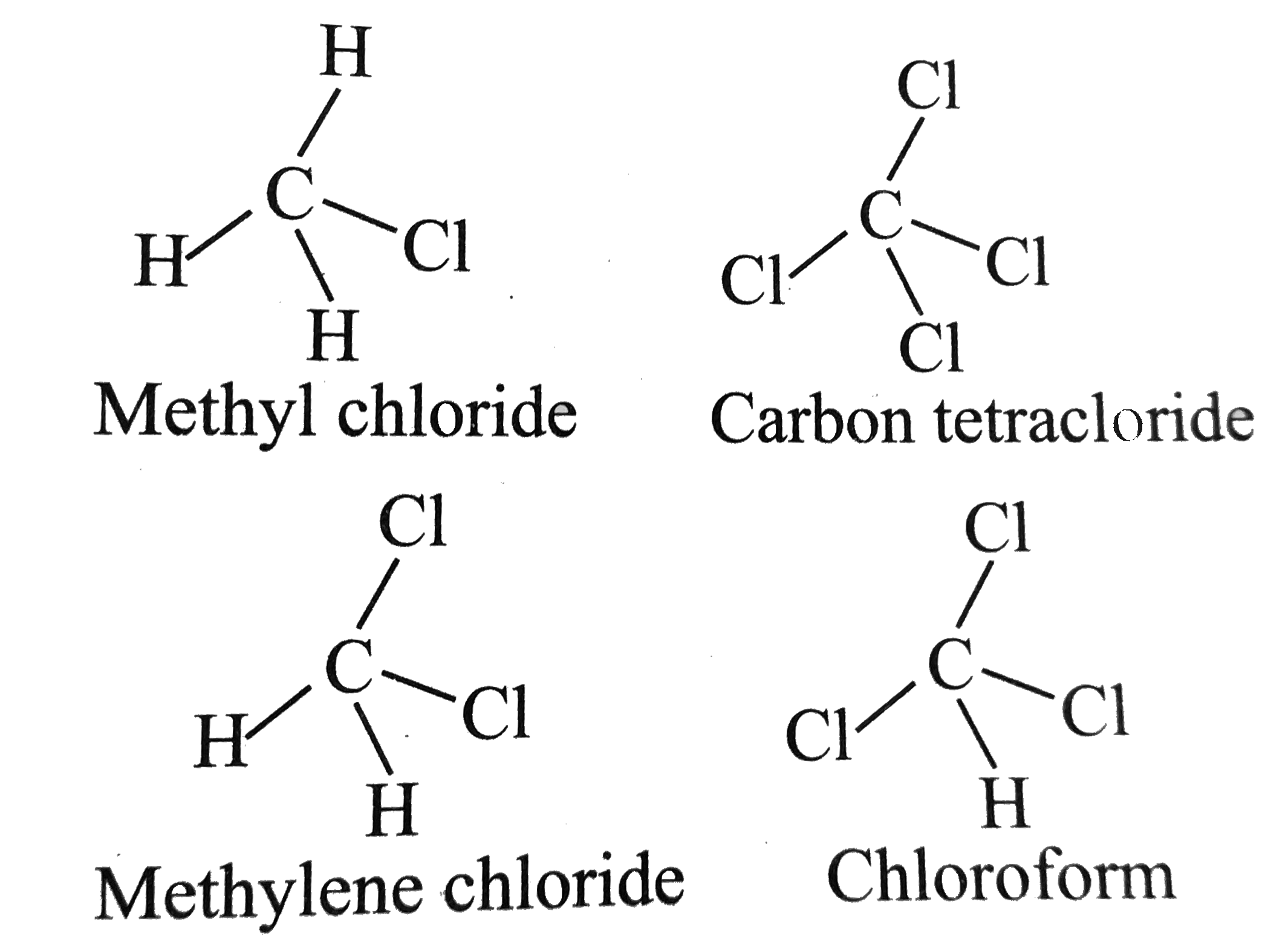
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY|Exercise Exercises Single Correct (Hybridisation)|33 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY|Exercise Exercises Single Correct (Bond Angle)|10 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY|Exercise Exercises Single Correct (Chemical Bonding)|46 VideosATOMIC STRUCTURE
CENGAGE CHEMISTRY|Exercise Concept Applicationexercise(4.3)|19 VideosCHEMICAL EQUILIBRIUM
CENGAGE CHEMISTRY|Exercise Archives (Subjective)|11 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-CHEMICAL BONDING AND MOLECULAR STRUCTURE-Exercises Single Correct (Dipole Moment)
- The H-O-H bond angle in the water molecule is 105^@ , theH -O bond dis...
Text Solution
|
- Diatomic molecule has a dipole moment of 1.2D If its bond 1.0 Å what f...
Text Solution
|
- The compound with no dipole moment is .
Text Solution
|
- The molecule which have zero dipole moments is .
Text Solution
|
- The critical temperature of water is higher than that of O(2) because ...
Text Solution
|
- The correct order of dipole moment is :
Text Solution
|
- Among the following which is polar ? .
Text Solution
|
- Which of the following is polar ? .
Text Solution
|
- The resultant dipole moment (mu) of two compounds NOF and NO(2)F is 1....
Text Solution
|
- In terms of polar character the correct, the correct order is .
Text Solution
|
- How many sigma and pi bonds are there in the molecule of tetracyano et...
Text Solution
|
- H(2)O is depolar, wheras BeF(2) is not. it because
Text Solution
|
- Which of the following hydrocarbons has the lowest dipole moment.
Text Solution
|
- Which one of the following arrangements of molecules is correct on the...
Text Solution
|
- Which statement (s) is/are correct about dipole momnet (I) Debye is ...
Text Solution
|
- Which of the following molecule (s) have dipole moment (I) Trans -pe...
Text Solution
|