A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY|Exercise Exercises Single Correct (Molecular Orbital Theory (Mot))|23 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY|Exercise Exercises Single Correct (Miscellaneous)|23 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY|Exercise Exercises Single Correct (Bond Angle)|10 VideosATOMIC STRUCTURE
CENGAGE CHEMISTRY|Exercise Concept Applicationexercise(4.3)|19 VideosCHEMICAL EQUILIBRIUM
CENGAGE CHEMISTRY|Exercise Archives (Subjective)|11 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-CHEMICAL BONDING AND MOLECULAR STRUCTURE-Exercises Single Correct (Resonance And Formal Charges)
- Which of the following conditions apply to resonating structrues ?
Text Solution
|
- Resonance structures can be written for .
Text Solution
|
- The bond length of C = O bond in CO is 1.20 Å and in CO(2) it is 1.34 ...
Text Solution
|
- Maximum number of H-bonds that can be formed by a water molecule is .
Text Solution
|
- Which of the following resonating structures is not correct for CO(2) ...
Text Solution
|
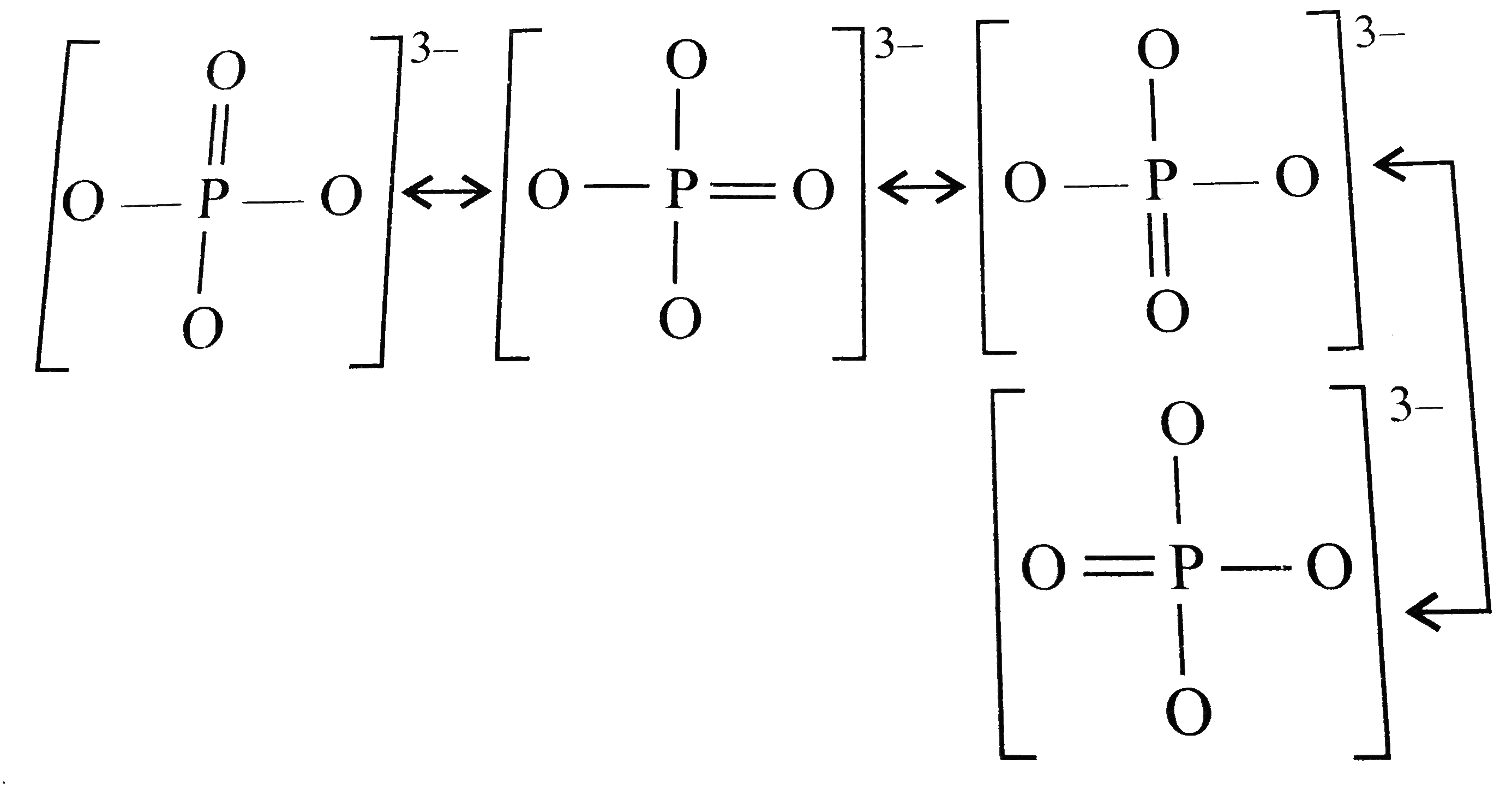
- In PO(4)^(3-) the formal charge on each O-atom and P-O bond order resp...
Text Solution
|
- The formed charge of the O-atoms in the ion [:overset(..)N=overset(..)...
Text Solution
|
- Which of the following statements regarding the concept of resonance i...
Text Solution
|
- Which of the following pairs do not constitute resonance structures ?
Text Solution
|
- Which of the following statement about resonance energy is wrong ? .
Text Solution
|
 .
.