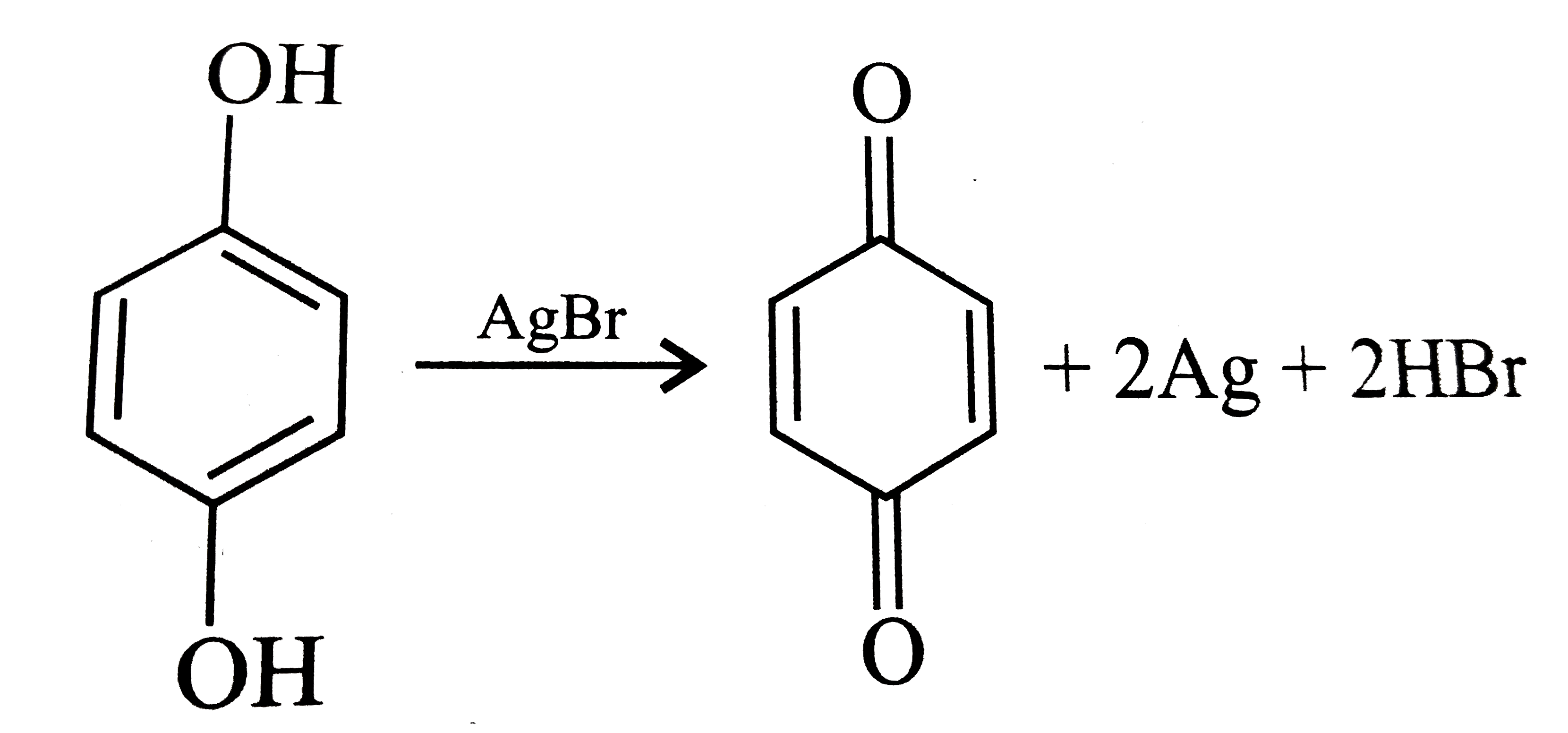
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-S-BLOCK GROUP 1 - ALKALI METALS-Archives Subjective
- Give reasons for the following: 'Sodium carbonate is prepared by Sol...
Text Solution
|
- Give balanced equations for the following: 'Carbon dioxide is passed...
Text Solution
|
- Write the balanced chemical equations for the following reactions. a...
Text Solution
|
- Complete and balance the following chemical reactions: anhydrous pot...
Text Solution
|
- Element (A) burns in nitrogen to give an ionic compound, (B) reacts wi...
Text Solution
|
- A white solid is either Na(2)O or Na(2)O(2). A piece of red litmus pap...
Text Solution
|
- Write the balanced chemical equation for developing photographic films...
Text Solution
|
- Identify the following: Na(2)CO(3)overset(So(2))rarr A overset(Na(2)...
Text Solution
|