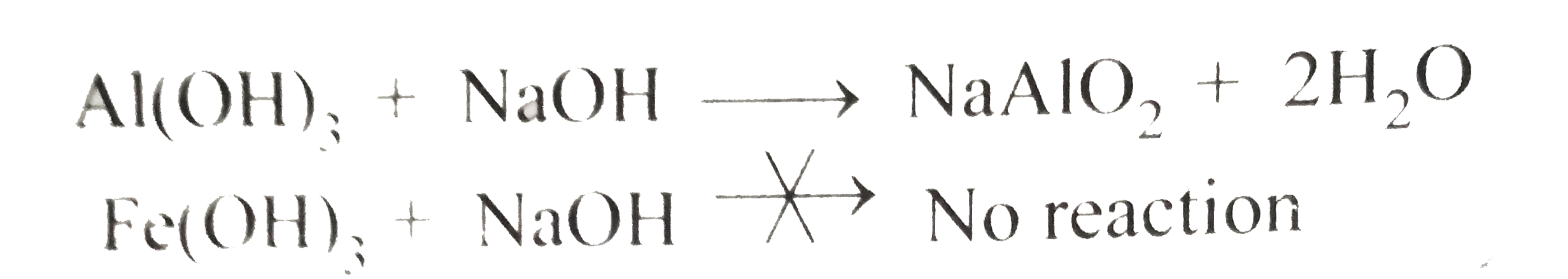Text Solution
Verified by Experts
Topper's Solved these Questions
P-BLOCK GROUP 13 - BORON FAMILY
CENGAGE CHEMISTRY|Exercise Solved Examples|16 VideosP-BLOCK GROUP 13 - BORON FAMILY
CENGAGE CHEMISTRY|Exercise Ex 6.1 (Subjective)|17 VideosORGANIC REACTION MECHANISM
CENGAGE CHEMISTRY|Exercise Analytical and Descriptive|6 VideosP-BLOCK GROUP 14 - CARBON FAMILY
CENGAGE CHEMISTRY|Exercise Exercises Archives (Subjective)|9 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-P-BLOCK GROUP 13 - BORON FAMILY-Exercise Archives (Subjecive)
- Give reasons for the following (a) No visible reaction occurs when a...
Text Solution
|
- State with balanced equations, what happens when aluminium is reacted ...
Text Solution
|
- State the conditions under which the preparation of aluminium is carri...
Text Solution
|
- Anhydrous AlCl(3) is covalent. From the date given below, predict whet...
Text Solution
|
- Aluminium sulphide gives a foul odour when it becomes damp. Write a ba...
Text Solution
|
- Write the chemical reactions associated with the 'borax' best 'test' o...
Text Solution
|
- Compound (X) on reduction with LiAlH(4) gives a hydride (Y) containing...
Text Solution
|
- How is boron obtained from borax ? Give the chemical reactions involve...
Text Solution
|
- Write the balanced equations for the reaction of the following compoun...
Text Solution
|
- AlF(3) is insoluble in anhydrous HF but dissolves on addition of NaF. ...
Text Solution
|