Text Solution
Verified by Experts
Topper's Solved these Questions
P-BLOCK GROUP 14 - CARBON FAMILY
CENGAGE CHEMISTRY|Exercise Solved Examples|9 VideosP-BLOCK GROUP 14 - CARBON FAMILY
CENGAGE CHEMISTRY|Exercise Ex (Subjective)|21 VideosP-BLOCK GROUP 13 - BORON FAMILY
CENGAGE CHEMISTRY|Exercise Exercise Archives (Subjecive)|9 VideosPERIODIC CLASSIFICATION OF ELEMENTS AND GENERAL INORGANIC CHEMISTRY
CENGAGE CHEMISTRY|Exercise Exercises (Archives )Subjective|4 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-P-BLOCK GROUP 14 - CARBON FAMILY-Exercises Archives (Subjective)
- Give reaasons : a. CCl4 is used as a fire extinguisher but not CS(2)...
Text Solution
|
- Write the chemical equations involved in the extraction of lead from g...
Text Solution
|
- State with balanced equations , what happens when .
Text Solution
|
- Give reason for the following in one or two sentences : ''Solid carbon...
Text Solution
|
- Give reasons for the following in one or two sentences : 'Graphite is ...
Text Solution
|
- Writebalanced equations for
Text Solution
|
- Complete the following reaction: Sn+2KOH+4H(2)Orarr……+…….
Text Solution
|
- Draw the structure of a cyclic silicate, (Si(3)O(9))^(6-) with proper ...
Text Solution
|
- Complete the reaction ltbr. SnCl4 + C2 H5 Cl+ Na rarr
Text Solution
|
- Starting from SiCl4 prepare the following in steps not exceeding the n...
Text Solution
|
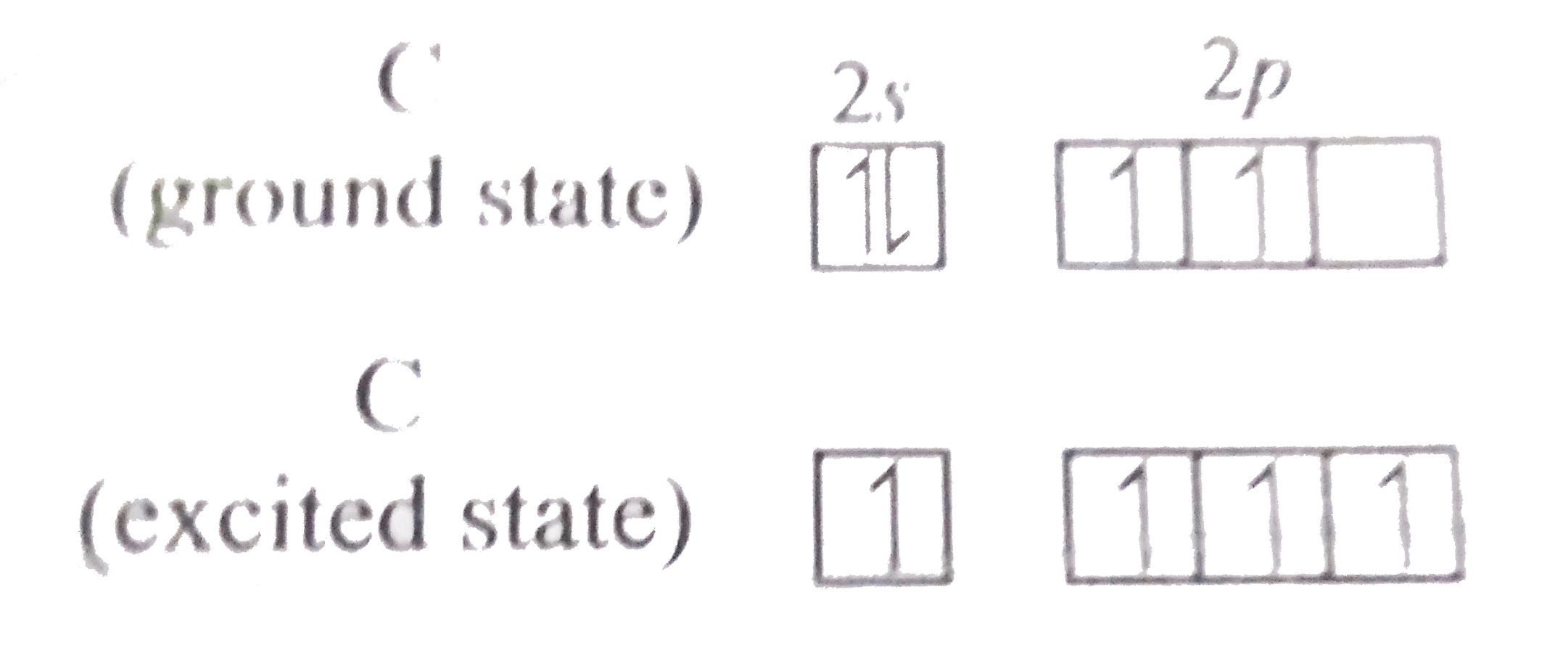
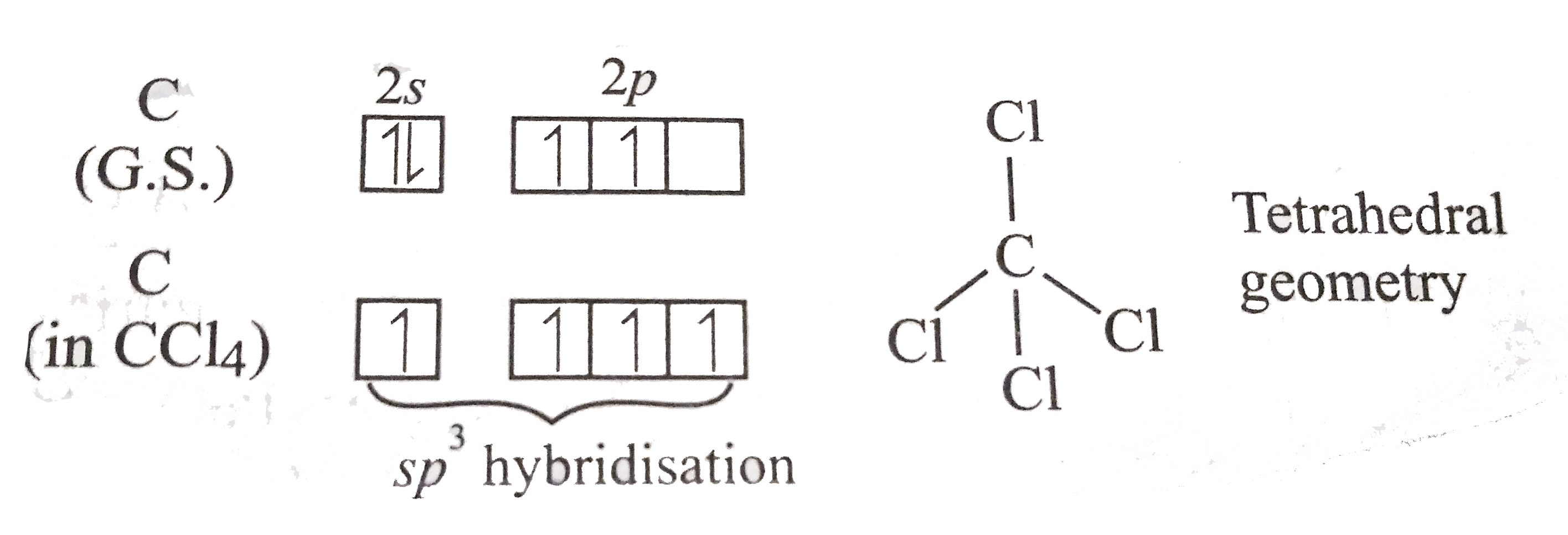 .
.