A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P-BLOCK GROUP 14 - CARBON FAMILY
CENGAGE CHEMISTRY|Exercise Exercises (Assertion-Reasoning)|27 VideosP-BLOCK GROUP 14 - CARBON FAMILY
CENGAGE CHEMISTRY|Exercise Exercises (Integer)|7 VideosP-BLOCK GROUP 14 - CARBON FAMILY
CENGAGE CHEMISTRY|Exercise Exercises (Multiple Correct )|19 VideosP-BLOCK GROUP 13 - BORON FAMILY
CENGAGE CHEMISTRY|Exercise Exercise Archives (Subjecive)|9 VideosPERIODIC CLASSIFICATION OF ELEMENTS AND GENERAL INORGANIC CHEMISTRY
CENGAGE CHEMISTRY|Exercise Exercises (Archives )Subjective|4 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-P-BLOCK GROUP 14 - CARBON FAMILY-Exercises (Single Correct)
- A when added to silica gives B. A and (B) are :
Text Solution
|
- A mong the following substitute stlanes the one which will give rese t...
Text Solution
|
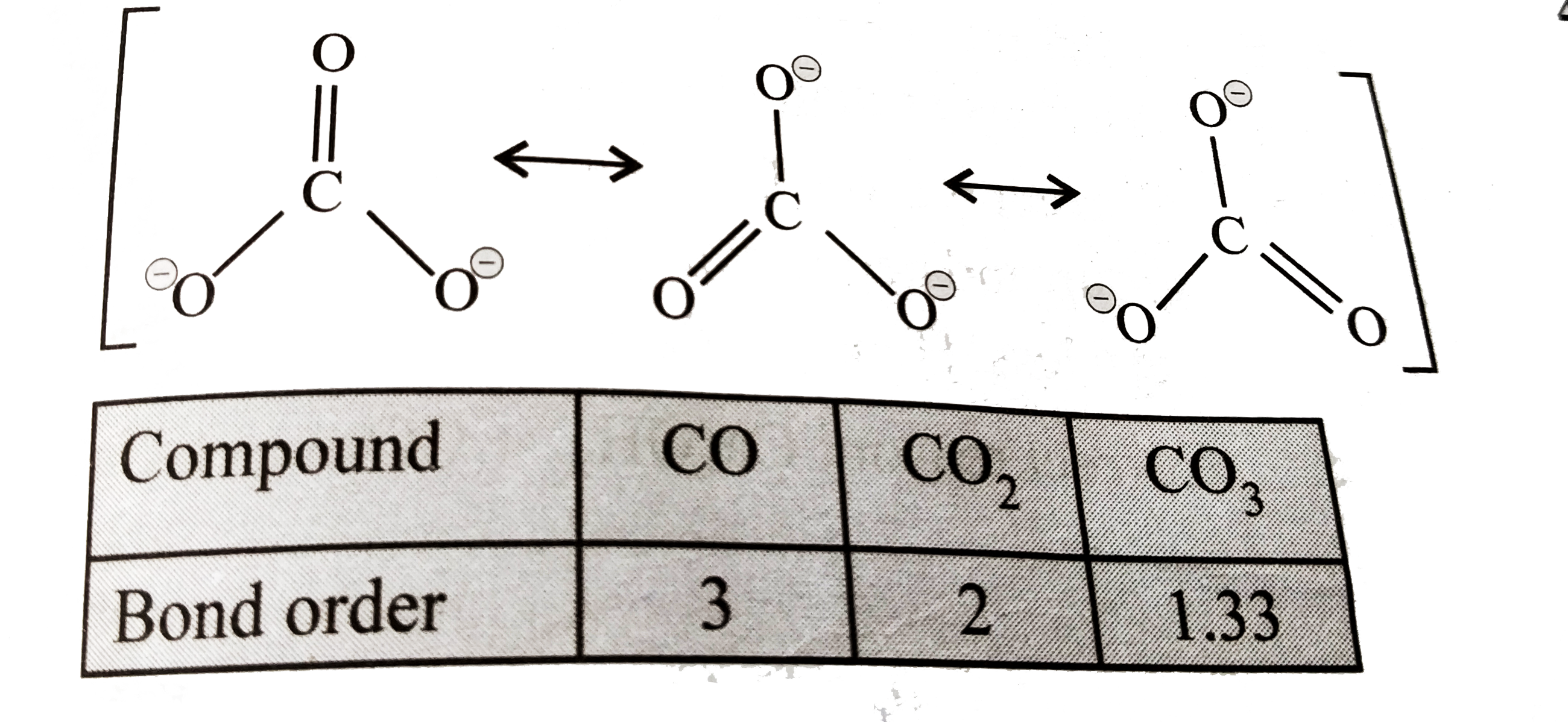
- The correct order of increasing C-O bond lengths in CO, CO3^(2-) and ...
Text Solution
|
- Which of the following has least tendncy to undegrgo catentaion ?
Text Solution
|
- which of the following statement is not correct ?
Text Solution
|
- On heating Pb(NO3)2 the products formed ate :
Text Solution
|
- The product of the following reaction are :
Text Solution
|
- in silicon dioxide :
Text Solution
|
- In the manufacture fo giass , the addition fo Mao2 gives ,
Text Solution
|
- Solder is an alloy of :
Text Solution
|
- Solder carbon sioxide is used as :
Text Solution
|
- Which gas is evolved when PbO(2) is treated with conc HNO(3) ?
Text Solution
|
- When steam reacts with red bot coke to form co2 and hydrogen :
Text Solution
|
- C Cl4 is used as fire extinguisher because :
Text Solution
|
- Lead dissolves most readily in
Text Solution
|
- Which of the following metals in an ujmportant ingredient fo transiont...
Text Solution
|
- The most unstable compounds fo the following are :
Text Solution
|
- Which of the following is most bsic ?
Text Solution
|
- The materical used in solar cells contains
Text Solution
|
- Softening of lead means:
Text Solution
|