Text Solution
Verified by Experts
Topper's Solved these Questions
APPENDIX - INORGANIC VOLUME 1
CENGAGE CHEMISTRY|Exercise chapter-2 Multiple correct answer|2 VideosAPPENDIX - INORGANIC VOLUME 1
CENGAGE CHEMISTRY|Exercise chapter-2 Single correct answer|3 VideosAPPENDIX - INORGANIC VOLUME 1
CENGAGE CHEMISTRY|Exercise Exercise very short|70 VideosALKYNES
CENGAGE CHEMISTRY|Exercise Exercises (Archives - Analytical and Desriptive Type)|4 VideosATOMIC STRUCTURE
CENGAGE CHEMISTRY|Exercise Concept Applicationexercise(4.3)|19 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-APPENDIX - INORGANIC VOLUME 1-Exercise Short
- The magnetic moment of KO(2) at room temperature is ---------- BM.
Text Solution
|
- On the basis of VSEPR theory, predict the shapes of the following mole...
Text Solution
|
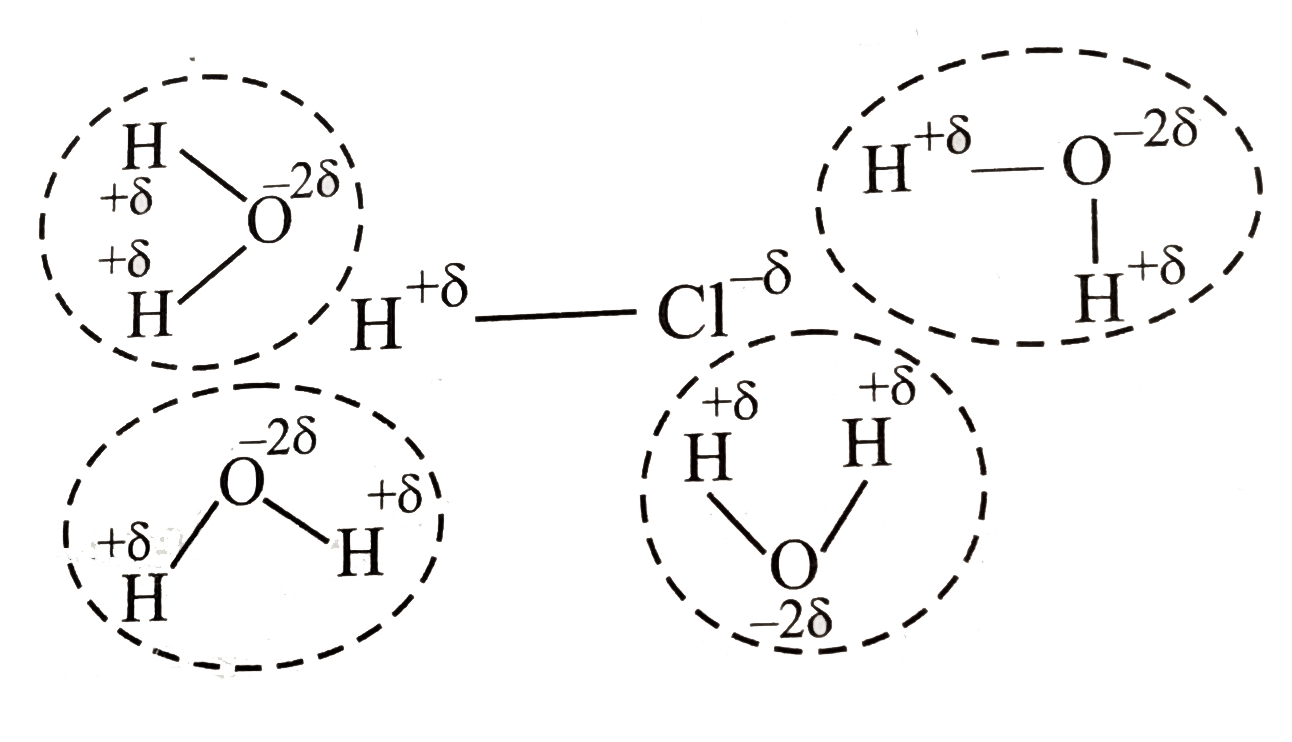
- Why is HCl predominantly covalent in the gaseous state but ionic in th...
Text Solution
|
- Why molbilities of H^(o+) ions in ice is greater as compared to liquid...
Text Solution
|
- Ionic bonds are non-directional while covalent bonds are directional.
Text Solution
|
- Write two resonance structure of N2 O that satisfy the octet rule.
Text Solution
|
- Whether molecular ion HeH^(Θ) exist or not? Explain.
Text Solution
|
- Out of but-1-yne or but-1-ene which has higher dipole moment?
Text Solution
|
- Write the structures of the following hydrates which contains ionic, c...
Text Solution
|
- What is formed when steam is passed over red hot coke?
Text Solution
|
- What is the name of the isotope of hydrogen which contains 1 proton an...
Text Solution
|
- Give the chemical reaction in which dihydrogen acts as an oxidising ag...
Text Solution
|
- Which element on treatment with caustic soda solution produces H(2) ga...
Text Solution
|
- What is meant by hardening of oils?
Text Solution
|
- Which gaseous compound on treatment with dihydrogen produces methanol?
Text Solution
|
- In order to produce pure dihydrogen gas, which combination is used?
Text Solution
|
- Assertion : Nascent hydrogen is more reactive than molecular hydrogen....
Text Solution
|
- What is the name given to hydrogen if nuclei of both the atoms have sa...
Text Solution
|
- what happens if conc H(2)SO(4) is used in preparing hydrogen by its re...
Text Solution
|
- A sample of hard water is allowed to pass through anion exchange resin...
Text Solution
|